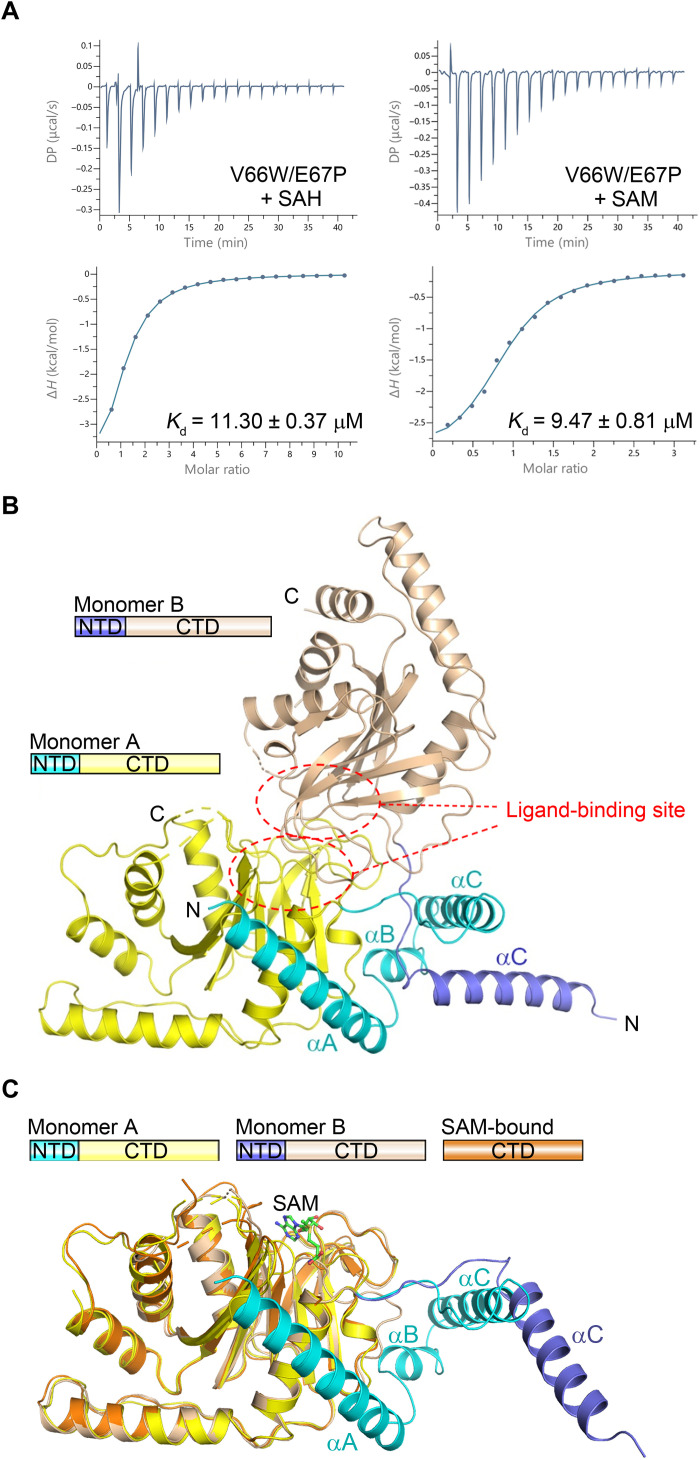
Fig. 2. The N-terminal domain of the dSAMTOR adopts multiple conformations.
(A) ITC measurements for the ligand-binding affinity of the V66W/E67P mutant dSAMTOR. The experiments were performed three times, which yielded similar results, and for each case, only the result of one representative experiment is shown. (B) Overall structure of the homodimer of the V66W/E67P mutant dSAMTOR in apo form. The two monomers are designated as monomer A and monomer B. The N-terminal helical domain (NTD) and the C-terminal MTase domain (CTD) of monomer A are colored in cyan and yellow, and those of monomer B are in blue and wheat, respectively. The ligand-binding site is indicated by dashed ovals. (C) Superposition of monomer A and monomer B in the V66W/E67P mutant dSAMTOR structure onto the SAM-bound MTase domain of dSAMTOR. The color coding of each structure is shown above.