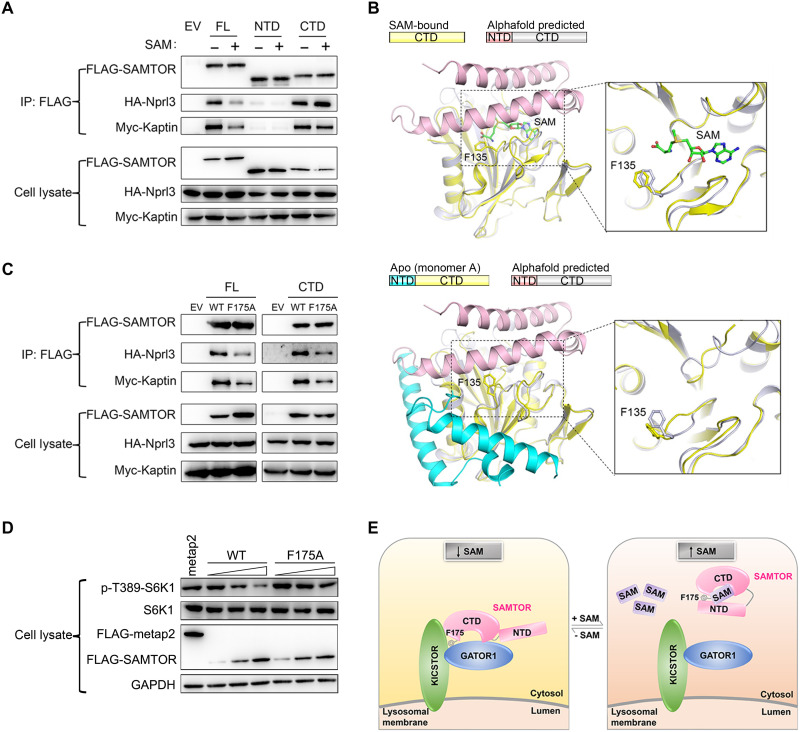
Fig. 4. The N-terminal domain functions as a molecular switch in mTORC1 signaling.
(A) Co-IP assay to examine the interactions of the full-length (FL), the N-terminal helical domain (NTD), and the C-terminal MTase domain (CTD) of hSAMTOR with HA-Nprl3 and Myc-Kaptin in the presence and absence of SAM in the HEK 293T cells. The assays in (A), (C), and (D) were performed three times, which yielded similar results, and for each case, only the result of one representative experiment is shown. (B) Structural comparison of the SAM-bound MTase domain of dSAMTOR (left) and the apo V66W/E67P mutant dSAMTOR (monomer A) (right) with the predicted dSAMTOR structure from the AlphaFold Protein Structure Database. The color coding of each structure is shown above. (C) Co-IP assay to examine the interactions of the WT and F175A mutant of the full-length (FL) and the C-terminal MTase domain (CTD) of hSAMTOR with HA-Nprl3 and Myc-Kaptin in the HEK 293T cells. (D) Immunoblotting assay to examine the mTORC1 kinase activity in the HEK 293T cells transiently overexpressing the WT and F175A mutant hSAMTOR at three different levels. The cell lysates were analyzed by immunoblotting of the phosphorylation level (p-T389) of S6K1 and the expression levels of the indicated proteins. (E) A schematic diagram illustrating the proposed molecular mechanism for SAMTOR to sense and bind SAM and then function as a molecular switch through conformational change to regulate its interaction with GATOR1-KICSTOR in mTORC1 signaling.