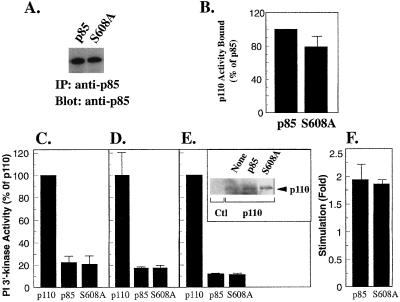
FIG. 3.
Inhibition of p110α by p85-S608A. (A) Expression of wild-type p85 and p85-S608A in Sf-9 lysates was measured by blotting with anti-p85 antibody. (B) Wild-type p85 and p85-S608A were immobilized on anti-p85–protein A-Sepharose beads, incubated with lysates from cells expressing N-myc-p110α, washed, and assayed for lipid kinase activity. Lipid kinase activity bound to wild-type p85 was defined as 100%. (C) Lysates from Sf-9 cells expressing N-myc-p110α were incubated in the absence or presence of wild-type p85 or p85-S608A and then assayed for lipid kinase activity (expressed as a percentage of lipid kinase activity in the absence of p85). (D) Lysates from Sf-9 cells expressing N-myc-p110α were incubated in the absence or presence of immunopurified wild-type p85 or p85-S608A and then assayed for lipid kinase activity (expressed as in panel C). (E) Sf-9 cells were infected with N-myc-p110α alone or were coinfected with wild-type p85 or p85-S608A. Lipid kinase activity in the cell lysates was determined (expressed as in panel C). Inset: p110α expression was determined by blotting with anti-p110α antibody. (F) Lysates containing N-myc-p110α and wild-type p85 or p85-S608A were incubated in the absence or presence of bisphosphopeptide (1 μM) for 1 h, and lipid kinase activity was determined. Activation was expressed as fold stimulation over activity in the absence of phosphopeptide. All determinations were made in duplicate or triplicate, and the data are the means ± standard errors of the means of four separate experiments.