Summary:
Discriminating dangerous predictive stimuli from non-threatening stimuli is vital for maintaining optimal behavioral strategies. A new study finds that novel stress-related peptide pathways to the dopaminergic midbrain play a fundamental role in threat generalization.
Animals respond to threats by engaging in defensive behaviors that promote safety at the expense of riskier alternatives, such as finding and exploiting resources. To maximize the odds of survival, an animal must discriminate threat-predictive cues from similar benign stimuli. Severe threats are associated with reduced discriminative accuracy, a phenomenon known as threat generalization1,2. Appropriately scaled threat generalization is adaptive; however, overgeneralization drives suboptimal behavioral policies and contributes to pathological fear and anxiety following traumatic stress in humans3. Aversive and threatening stimuli elicit secretion of dynorphins, the endogenous peptide ligands of the kappa opioid receptor (KOR), and KOR signaling is implicated in aversive emotional and physiological states4. A robust body of literature has established KOR signaling in dopamine neurons of the midbrain ventral tegmental area (VTA) as a critical mediator of anxiety, avoidance, and dysphoria5. Dopamine released from VTA neurons is necessary for both the acquisition of threat associations and the representation of threat probability6,7; however, it was previously unclear how KOR activation in high-threat conditions affects threat representation and subsequent behavioral responses. A new study by Fellinger et al8 investigates novel dynorphin pathways to the VTA and reveals a role of KOR-mediated regulation of dopamine neurons in threat generalization.
To study threat generalization, Fellinger et al used a Pavlovian conditioning paradigm in which mice learn to discriminate an auditory cue that is paired with footshock (the CS+) from a distinct auditory cue with no adverse outcome (the CS−). Following two days of conditioning, discrimination was assayed with measurements of defensive freezing behavior elicited by the CS+ and CS−. By varying the intensity of the footshock during conditioning, the authors demonstrate that threat discrimination has an inverted U-shaped relationship with shock intensity, with animals displaying generalized freezing behavior to both the CS+ and CS− as shock intensity increases. This manifestation of the Yerkes-Dodson law9, which describes a parabolic relationship between stressor intensity and behavioral performance, has been observed in numerous other species including rats, fish, and humans1,2,10,11. Because footshock stress activates KORs, and stress-evoked aversive behaviors require KOR signaling4,12, the authors next antagonized KORs by injecting the long-acting drug norBNI prior to threat conditioning. This pharmacological treatment did not alter shock responsivity or freezing behavior during conditioning, but it did reduce freezing to the CS− during discrimination testing in mice conditioned at higher footshock intensities, linking the KOR to threat generalization.
Prior work demonstrated that VTA dopamine neurons show either increased or decreased activity in response to threats, and the relative activity of these neurons to the CS+ and CS− corresponds to fear discrimination behavior7. Fellinger et al therefore hypothesized that VTA dopamine neurons may be a critical site of KOR signaling for threat generalization. To test this, the authors employed an innovative gene editing strategy using CRISPR/Cas9 technology. They injected a conditional adeno-associated viral vector expressing SaCas9 and a single-guide RNA into the VTA to induce mutagenesis of the gene encoding the KOR (Oprk1) specifically in dopamine neurons. Selective mutagenesis of Oprk1 in VTA dopamine neurons substantially attenuated threat generalization by decreasing freezing responses to the CS− during discrimination testing.
Fellinger et al next sought to determine the potential sources of dynorphin to the midbrain. To achieve this, they used an elegant viral vector-based neuroanatomical tracing strategy in a transgenic mouse model to allow identification of neurons expressing the preprodynorphin gene. This method revealed previously unexplored preprodynorphin-expressing projections to the VTA originating from the bed nucleus of the stria terminalis (BNST) and the dorsal raphe nucleus (DRN). Surprisingly, the authors did not report dynorphin input to the VTA from the lateral hypothalamus, as has been characterized previously13; however, this may be due to the selectivity or efficacy of different tracing strategies. Using a similar method in a conditional knockout mouse model, they then deleted the preprodynorphin gene in VTA-projecting neurons, which resulted in reduced CS− freezing during the discrimination test. Together with the results of the Oprk1 mutagenesis experiments, this supports the hypothesis that dynorphin acting at KORs in VTA dopamine neurons is necessary for generalization.
To determine the relative contribution of the BNST and DRN pathways in threat discrimination, Fellinger et al employed optogenetics to stimulate the axon terminals of preprodynorphin-expessing BNST or DRN neurons in the VTA. Mice were conditioned using a low shock intensity and axon terminals were optogenetically stimulated during the shock. Stimulation of BNST terminals increased freezing behavior to both the CS+ and CS− compared to controls, but this stimulation did not alter the threat discrimination ratio. In contrast, activating the axon terminals of the DRN projection promoted generalization by elevating conditioned freezing responses specifically to the CS−. These experiments identify and establish the preprodynorphin-expressing DRN input to the VTA as a critical effector of threat-sensitive generalization. Interestingly, activating both the DRN and the BNST inputs to VTA increased anxiety-like behavior as measured by time in the open arms of the elevated plus maze. These results provide important insight to the dissociable neural processes mediating threat generalization among discrete cues versus the cue-independent hypervigilance state of anxiety.
In sum, Fellinger et al’s findings connect the parallel literature of dopaminergic threat representations and aversive dynorphin/KOR signaling in the dopaminergic midbrain by demonstrating a crucial role for a novel, discrete dynorphin input to VTA dopamine neurons in generalization. It is important to note that separate populations of VTA dopamine neurons send their axons to distinct brain regions14, and dynorphin differentially regulates the activity of these subpopulations5. Fellinger et al find that BNST and DRN inputs target different subregions of the VTA, which may explain the differential impact on behavior mediated by these pathways. Further studies to determine the downstream targets of VTA dopamine neurons receiving dynorphin input from the BNST versus DRN would clarify our understanding of the functional organization of the VTA.
Prior work suggests that VTA projections to the lateral amygdala and central nucleus of the amygdala influence threat generalization. Neuronal activity in the lateral amygdala correlates with behavioral threat discrimination, with activity becoming nonspecific as generalization develops15,16. Genetic manipulations that reduce phasic bursting activity of VTA dopamine neurons impair discriminative coding and promote generalization, with the caveat that generalization occurs via reduced responding to the CS+, whereas DRN dynorphin-mediated inhibition of dopamine neurons increased responding to the CS−17. Circuit dynamics in the central amygdala also control threat discrimination, and this circuitry is sensitive to dopaminergic antagonism18,19. Threat generalization gradients can be bidirectionally manipulated by increasing or decreasing the activity of central amygdala dopamine terminals during the CS+, and generalization here is mediated by increased responding to the CS−7. Dynorphin may therefore promote generalization by inhibiting transmission from central amygdala-projecting dopamine neurons.
It has been proposed that in the lateral amygdala the stress of a high-intensity footshock results in the CS+ and CS− being compounded into a single neuronal representation that is 50% predictive of shock16. Fellinger et al’s observation of selective enhancement of freezing to the CS− without concomitant reduction in freezing to the CS+ is inconsistent with this idea. Additionally, generalization may not solely be related to altered associative learning in the midbrain, because the dopaminergic representation of cues is not static. Rather, cue representations are modulated by physiological state and the context in which they are experienced. Indeed, the stress of experiencing the CS+ may acutely influence representation of the CS−, a possibility which could be resolved by testing recall of each cue in independent sessions. Furthermore, it is unclear if this stress- and dopamine-dependent mechanism of threat generalization also mediates instances of generalization that cannot be explained by altered learning. In post-traumatic stress disorder, generalization of threat responses to novel stimuli, rather than stimuli experienced during the traumatic event, is especially problematic. Acute stress also induces generalization among previously discriminated cues20. Further work is needed to probe the role of KOR regulation of dopamine signals in these state-dependent shifts in threat discrimination.
Figure 1. Novel dynorphin inputs to VTA dopamine neurons regulate threat discrimination and anxiety-like behavior.
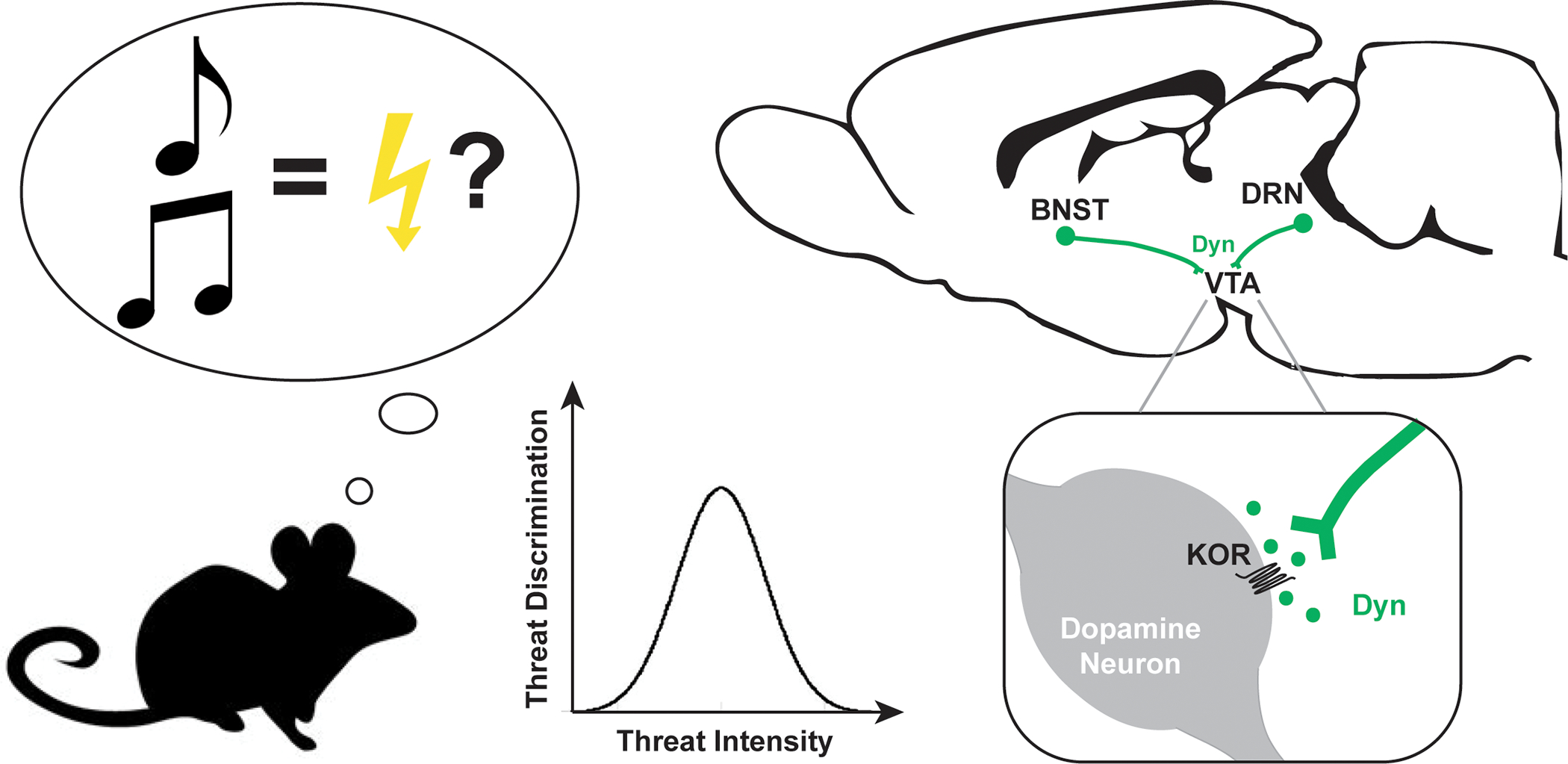
Associative memories guide the behavioral response to previously encountered stimuli. Behavioral discrimination between cues that were paired with shock and cues that were not shock-predictive follows an inverted U-shaped pattern, with optimal discrimination at moderate threat intensities and generalization at high intensities. Fellinger et al demonstrate that threat generalization requires dynorphin signaling at kappa opioid receptors in the ventral tegmental area (VTA). Tracing studies revealed previously unidentified dynorphin inputs to the VTA from the bed nucleus of the stria terminalis (BNST) and the dorsal raphe (DRN). Activating either of these dynorphin projections is sufficient to induce anxiety-like behavior; however, only activation of the DRN projection promotes threat generalization.
References:
- 1.Laxmi TR, Stork O, and Pape HC (2003). Generalisation of conditioned fear and its behavioural expression in mice. Behav Brain Res 145, 89–98. [DOI] [PubMed] [Google Scholar]
- 2.Baldi E, Lorenzini CA, and Bucherelli C (2004). Footshock intensity and generalization in contextual and auditory-cued fear conditioning in the rat. Neurobiol Learn Mem 81, 162–166. [DOI] [PubMed] [Google Scholar]
- 3.Elzinga BM, and Bremner JD (2002). Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? J Affect Disord 70, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, and Chavkin C (2008). The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci 28, 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Margolis EB, and Karkhanis AN (2019). Dopaminergic cellular and circuit contributions to kappa opioid receptor mediated aversion. Neurochem Int 129, 104504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fadok JP, Dickerson TM, and Palmiter RD (2009). Dopamine is necessary for cue-dependent fear conditioning. J Neurosci 29, 11089–11097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jo YS, Heymann G, and Zweifel LS (2018). Dopamine Neurons Reflect the Uncertainty in Fear Generalization. Neuron 100, 916–925 e913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fellinger L, Yong S, Hunker AC, Soden ME, Juarez B, Elum J, and Zweifel LS (2021). A midbrain dynorphin circuit promotes threat generalization. Current Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yerkes RM, and Dodson JD (1908). The relation of strength of stimulus to the rapidity of habit-formation. Journal of Comparative Neurology and Psychology 18, 459–482. [Google Scholar]
- 10.Ferrari MC, Messier F, and Chivers DP (2008). Can prey exhibit threat-sensitive generalization of predator recognition? Extending the Predator Recognition Continuum Hypothesis. Proc Biol Sci 275, 1811–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunsmoor JE, Kroes MCW, Braren SH, and Phelps EA (2017). Threat intensity widens fear generalization gradients. Behav Neurosci 131, 168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLaughlin JP, Xu M, Mackie K, and Chavkin C (2003). Phosphorylation of a carboxyl-terminal serine within the kappa-opioid receptor produces desensitization and internalization. J Biol Chem 278, 34631–34640. [DOI] [PubMed] [Google Scholar]
- 13.Fallon JH, Leslie FM, and Cone RI (1985). Dynorphin-containing pathways in the substantia nigra and ventral tegmentum: a double labeling study using combined immunofluorescence and retrograde tracing. Neuropeptides 5, 457–460. [DOI] [PubMed] [Google Scholar]
- 14.Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, Gao XJ, Kremer EJ, Malenka RC, and Luo L (2015). Circuit Architecture of VTA Dopamine Neurons Revealed by Systematic Input-Output Mapping. Cell 162, 622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins DR, and Pare D (2000). Differential fear conditioning induces reciprocal changes in the sensory responses of lateral amygdala neurons to the CS(+) and CS(−). Learn Mem 7, 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh S, and Chattarji S (2015). Neuronal encoding of the switch from specific to generalized fear. Nat Neurosci 18, 112–120. [DOI] [PubMed] [Google Scholar]
- 17.Jones GL, Soden ME, Knakal CR, Lee H, Chung AS, Merriam EB, and Zweifel LS (2015). A genetic link between discriminative fear coding by the lateral amygdala, dopamine, and fear generalization. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Botta P, Demmou L, Kasugai Y, Markovic M, Xu C, Fadok JP, Lu T, Poe MM, Xu L, Cook JM, et al. (2015). Regulating anxiety with extrasynaptic inhibition. Nat Neurosci 18, 1493–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Bundel D, Zussy C, Espallergues J, Gerfen CR, Girault JA, and Valjent E (2016). Dopamine D2 receptors gate generalization of conditioned threat responses through mTORC1 signaling in the extended amygdala. Mol Psychiatry 21, 1545–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunsmoor JE, Otto AR, and Phelps EA (2017). Stress promotes generalization of older but not recent threat memories. Proc Natl Acad Sci U S A 114, 9218–9223. [DOI] [PMC free article] [PubMed] [Google Scholar]


