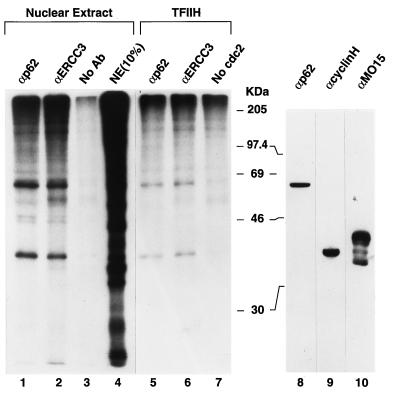
FIG. 5.
TFIIH polypeptides are phosphorylated in vitro by mitotic kinases. HeLa cell nuclear extract (10 μg of protein per 20-μl reaction volume) was treated with mitotic kinases bound to p13-agarose beads (20 μl per reaction) and [γ-32P]ATP for 1 h at ambient temperature. After a brief centrifugation to remove the agarose beads, the reaction volume was brought to 100 μl with PBS and TFIIH was immunoprecipitated with protein A-Sepharose beads (25 μl per reaction) prebound with 2.5 μl of rabbit polyclonal serum to the p62 (lane 1) and ERCC3 (lane 2) subunits of TFIIH (αp62 and αERCC3, respectively) or no antibody (No Ab) (lane 3). The immunoprecipitates were washed three times with 150 μl of PBS containing 0.1% (vol/vol) Triton X-100, and precipitated proteins were eluted from the Sepharose beads with SDS sample buffer. Lane 4 shows a 1/10-volume aliquot of the labeled nuclear extract proteins (NE) prior to immunoprecipitation. Biochemically purified TFIIH (600 ng of protein) was phosphorylated with p13-bound kinase, as described above, and after removal of the kinase, TFIIH was immunoprecipitated with anti-p62 antibody (lane 5) or anti-ERCC3 antibody (lane 6) and analyzed as described above. Autophosphorylation of TFIIH is shown in lane 7. The autoradiogram of an SDS-10% PAGE gel is shown. A Western blot of the HeLa cell nuclear extract was probed with the indicated antibodies to TFIIH (anti-p62, anti-cyclin H, and anti-MO15) subunits (lanes 8 to 10, respectively). The positions of molecular mass markers (in kilodaltons) are indicated.