1.
Plasmablastic lymphoma (PBL) is a rare and aggressive subtype of large B‐cell lymphoma with plasmacytic differentiation. Although limited‐stage (LS) PBL has a favorable outcome, extensive‐stage (ES) PBL has a poor prognosis, with a median survival of 12–18 months [1, 2, 3]. Newly diagnosed PBL is commonly treated with combination chemotherapy, such as dose‐adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (DA‐EPOCH), followed by consolidation autologous stem cell transplant (autoSCT) in ES cases [2, 4]. Novel agents such as bortezomib, lenalidomide, and daratumumab have been used in case reports or small case series, often as a component of multi‐agent therapy in both the frontline and salvage settings [5, 6]. However, due to small sample sizes and a lack of prospective studies, the clinical benefit of these agents are not well established. Patients with relapsed/refractory (r/r) PBL still have a very poor outcome and most die from the disease.
B‐cell maturation antigen (BCMA) is a cell surface protein involved in B‐cell maturation and differentiation into plasma cells. Physiologically, BCMA is exclusively expressed in mature plasma cells and a subset of B‐cells. Multiple myeloma (MM) cells universally express BCMA [7]. BCMA‐directed cellular and immunotherapies are emerging as important treatment options for r/r MM [8, 9]. Studies with small sample sizes reported variable levels of BCMA expression in Hodgkin lymphoma and non‐Hodgkin B‐cell lymphoma [10]. However, most of these studies did not evaluate PBL as a separate group. One study reported seven PBL cases and all of them had strong BCMA expression rated as 3+ on immunohistochemistry (IHC) [11]. In the current study, we identified all patients with a diagnosis of PBL who presented to Moffitt Cancer Center in Florida, USA from January 2006 to April 2023, and retrospectively performed BCMA IHC staining (Cell signaling Technology clone E6D7B, Leica BOND IHC Protocol) on the available archived FFPE biopsy samples. The study was approved by the institutional review boards.
Progression‐free survival (PFS) was calculated as the time from disease progression or last follow‐up to treatment start date. Overall survival (OS) was calculated as the time from death or last follow‐up to date of PBL diagnosis. The association of BCMA expression with PFS or OS was evaluated using a log‐rank test, with continuous variables converted to categorical by classifying them as above or below the median. All analyses were done using SAS 9.2.
A total of 32 PBL patients were identified. The median age was 59 years (range 31–83 years); 11 patients (34.4%) were in stage I/II (LS) and 21 (65.6%) had stage III/IV (ES); nine patients (28.1%) were HIV positive; and 57.1% of evaluated cases were EBV positive by EBER. Treatment data was available for 30 patients. The most common frontline therapies were EPOCH and CHOP with or without rituximab; four patients received bortezomib combined with EPOCH; seven patients (23.3%) received autoSCT in the first remission (Table 1). Median follow‐up was 77.6 months calculated using the reverse Kaplan‐Meier method. Consistent with previous reports, patients with ES PBL had worse outcome than those with LS PBL, with 3‐year OS rates of 24% and 100%, respectively, p = 0.01 (Table 1).
TABLE 1.
Patient characteristics, treatments, outcomes and B‐cell maturation antigen (BCMA) expression in plasmablastic lymphoma (PBL).
| Number (%) | Extensive stage (ES, III‐IV) | Limited stage (LS, I‐II) | ALL patients |
|---|---|---|---|
| N | 21 | 11 | 32 |
| Age, years, Median (range) | 55 (31–81) | 60 (38–83) | 59 (31–83) |
| Male | 13 (61.9) | 9 (81.8) | 22 (68.8) |
| ECOG performance status | |||
| < / = 2 | 18 (85.7) | 11 (100) | 29 (90.6) |
| > 2 | 3 (14.3) | 0 (0.0) | 3 (9.4) |
| HIV positive | 9 (42.9) | 0 (0.0) | 9 (28.1) |
| EBV positive | 10/18 (55.6) | 6/10 (60) | 16/28 (57.1) |
| MYC rearrangement | 5/10 (50.0) | 0/4 (0.0) | 5/14 (35.7) |
| MYC and BCL2 rearrangement | 0/10 (0.0) | 0/4 (0.0) | 0/14 (0.0) |
| CD20 positive | 4/20 (20.0) | 1/10 (10.0) | 5/30 (16.7) |
| CD19 positive | 2/9 (22.2) | 1/3 (25.0) | 3/13 (23.1) |
| Frontline treatment | |||
| CHOP +/‐ R | 5 (23.8) | 5 (55.6) | 10 (33.3) |
| EPOCH +/‐ R | 11 (52.4) | 2 (22.2) | 13 (43.3) |
| EPOCH + bortezomib +/‐ R | 4 (19.1) | 1 (11.1) | 5 (16.7) |
| Other* | 1 (4.8) | 1 (11.1) | 2 (6.7) |
| Radiation therapy | 6 (28.6) | 4 (44.4) | 10 (33.3) |
| Autologous SCT | 6 (28.6) | 1 (11.1) | 7 (23.3) |
| Allogeneic SCT | 2 (9.5) | 0 (0.0) | 2 (6.3) |
| CD19 CAR‐T | 2/21 (9.5) | 0/9 (0.0) | 2/30 (6.7) |
| Response to frontline treatment | |||
| CR | 9 (45.0) | 7 (87.5) | 16 (57.1) |
| PR | 3 (15.0) | 1 (12.5) | 4 (14.3) |
| SD/PD | 8 (40.0) | 0 (0.0) | 8 (28.6) |
| PFS | P value** | ||
| Median (95%CI), months | 6.7 (3.4, 8.3) | NR (NA) | 0.002 |
| 3‐year PFS rate | 21.2% | 100% | |
| OS | P value** | ||
| Median (95%CI), months | 14.7 (10.2, 33.7) | 89.1 (56.7, NA) | 0.01 |
| 3‐year OS rate | 24.0% | 100% | |
| BCMA Immunohistochemistry | Extensive stage (III‐IV) | Limited stage (I‐II) | ALL patients |
| N | 13 | 5 | 18 |
| Proportion of BCMA+ cells, median (IQR) | 70% (5%–100%) | 90% (40%, 100%) | 75% (40%, 100%) |
| BCMA expression strength, N (%) | |||
| Negative | 1 (7.7) | 0 (0) | 1 (5.6) |
| Weak | 5 (38.5) | 1 (20) | 6 (33.3) |
| Intermediate to strong | 7 (53.8) | 4 (80.0) | 11 (61.1) |
Abbreviations: BCMA, B‐cell maturation antigen; CHOP, cyclophosphamide, doxorubicin, vincristine and prednisone; CI, confidence interval; CR, complete recovery; EPOCH, etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin; LS, limit stage; NA, not estimable; NR, not reached; OS, overall survival; PFS, progression‐free survival; PR, partial recovery; SCT, stem cell transplant.
Two patients in LS missed data on treatment; Three patients in LS and one patient in ES missed data on treatment responses.
One patient received hyper‐CVAD, and another patient received CyBorD (cyclophosphamide, bortezomib, and dexamethasone).
p‐Value calculated using log‐rank test.
Among the 20 patients with ES PBL and known treatment responses, 11 did not achieve complete recovery (CR) after frontline therapy. Ten of these 11 patients died, nine within 2 years, and one within 3 years; the only patient who survived had a very good partial response to frontline CHOP and proceeded with autoSCT.
Two patients with CD19+ PBL received CD19 Chimeric antigen receptor (CAR)‐T after multiple lines of therapy. Both died within 30 days, one from disease progression and the other from multiple treatment complications.
Out of the 18 cases with tissue available for the IHC study, 17 (94.4%) had positive BCMA expression (Figure 1A). The median percentage of BCMA+ cells was 75%. Eleven (61.1%) cases had intermediate to strong BCMA staining (Figure 1B). Neither BCMA+ cell percentage nor BCMA expression level was associated with disease stage, response rate, PFS, or OS (p > 0.10 for all tests).
FIGURE 1.
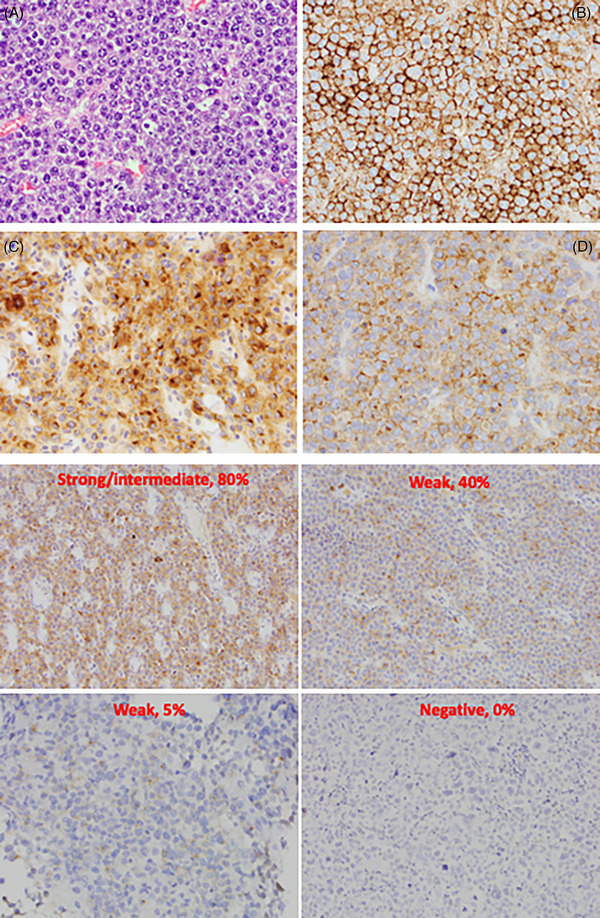
a: Images (40X) of the neoplastic plasmablastic lymphoma (PBL) cells H & E (A), immunohistochemical stain for CD138 identifying the PBL cells (B), B‐cell maturation antigen (BCMA) cytoplasmic staining pattern (C), and BCMA membranous staining pattern (D). b: Representative intensities and percentages of BCMA expression.
In our study, the majority of our PBL cases tested positive for BCMA, and the percentage of BCMA+ cells seemed comparable to MM cases [7]. To our knowledge, this is the largest and the first report that systemically evaluated BCMA expression in PBL. Our results provide evidence to explore BCMA as a potential target in PBL. Targeting BCMA demonstrated important success in MM with two anti‐BCMA CAR‐T products, idecabtagene vicleucal (ide‐cel) and ciltacabtagene autoleucel (cilta‐cel), currently approved for r/r MM after four or more lines of therapy [8, 9]. Similarly, the anti‐BCMA/CD3 bispecific T‐cell engager, teclistamab, was also granted approval for r/r MM [12]. Belantamab mafodotin‐blmf, an antibody‐drug conjugate targeting BCMA was initially granted accelerated approval by the Food and Drug Administration for r/r MM but was voluntarily withdrawn from the market upon negative results of the phase 3 DREAMM‐3 trial. Given the expression of BCMA in PBL and the lack of effective treatment of r/r PBL, these patients could be considered in trials evaluating the BCMA‐targeting therapies. In fact, in a case report recently published, one patient with refractory PBL achieved CR after one infusion of cilta‐cel and remained in CR after 14 months [13]. Another noteworthy point in our study is, 10 of our 11 ES patients who did not achieve CR after frontline chemotherapy died from the disease, therefore clinical trials for this condition are of paramount importance, especially with consideration of BCMA‐directed therapies. Besides IHC, flow cytometry can be used to detect and quantified BCMA expression and can be used in clinical practice [14]. Lastly, we did not find an association of BCMA expression with clinical outcomes as oppose of MM, in which higher BCMA expression was associated with shorter PFS and OS [7]. The lack of association in our study may be due to the small sample size, or, it may reflect a difference in biological role of BCMA in PBL from MM. Our results will need to be corroborated with other series, in order to confirm our findings. Translational studies need to evaluate the dynamics of BCMA protein expression on PBL cell membrane and the activity of BCMA‐targeting agents in vitro and in animal models of PBL, exploring the potential difference of their activities in PBL and MM.
AUTHOR CONTRIBUTIONS
Ning Dong, Hailing Zhang, Jinming Song, and Julio Chavez designed the study. Ning Dong, Hailing Zhang, Jinming Song, and Jamila Mammadova acquired the data. Ning Dong, Hailing Zhang, and Jinming Song analyzed the data. Ning Dong and Julio Chavez wrote the article and all other authors contributed to the draft. All authors have given final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING INFORMATION
This study was not funded.
ETHICS STATEMENT
The study was approved by the institutional review boards (IRB).
PATIENT CONSENT STATEMENT
A waiver of Consent and Waiver of HIPAA authorization was granted by the IRB.
CLINICAL TRIAL REGISTRATION
The authors have confirmed clinical trial registration is not needed for this submission.
Dong N, Zhang H, Song J. B‐cell maturation antigen expression and clinical features of plasmablastic lymphoma. eJHaem. 2024;5:285–289. 10.1002/jha2.807
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Hess BT, Giri A, Park Y, Patel KK, Link BK, Nowakowski GS, et al. Outcomes of patients with limited‐stage plasmablastic lymphoma: a multi‐institutional retrospective study. Am J Hematol. 2023;98(2):300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood 2015;125(15):2323–2330. [DOI] [PubMed] [Google Scholar]
- 3. Liu JJ, Zhang L, Ayala E, Field T, Ochoa‐Bayona JL, Perez L, et al. Human immunodeficiency virus (HIV)‐negative plasmablastic lymphoma: a single institutional experience and literature review. Leuk Res. 2011;35(12):1571–1577. [DOI] [PubMed] [Google Scholar]
- 4. Makady NF, Ramzy D, Ghaly R, Abdel‐Malek RR, Shohdy KS. The emerging treatment options of plasmablastic lymphoma: analysis of 173 individual patient outcomes. Clin Lymphoma Myeloma Leuk. 2021;21(3):e255–e263. [DOI] [PubMed] [Google Scholar]
- 5. Castillo JJ, Guerrero‐Garcia T, Baldini F, Tchernonog E, Cartron G, Ninkovic S, et al. Bortezomib plus EPOCH is effective as frontline treatment in patients with plasmablastic lymphoma. Br J Haematol. 2019;184(4):679–682. [DOI] [PubMed] [Google Scholar]
- 6. Dittus C, Miller JA, Wehbie R, Castillo JJ. Daratumumab with ifosfamide, carboplatin and etoposide for the treatment of relapsed plasmablastic lymphoma. Br J Haematol. 2022;198(2):e32–e34. [DOI] [PubMed] [Google Scholar]
- 7. Lee L, Bounds D, Paterson J, Herledan G, Sully K, Seestaller‐Wehr LM, et al. Evaluation of B cell maturation antigen as a target for antibody drug conjugate mediated cytotoxicity in multiple myeloma. Br J Haematol. 2016;174(6):911–922. [DOI] [PubMed] [Google Scholar]
- 8. Munshi NC, Anderson LD, Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705–716. [DOI] [PubMed] [Google Scholar]
- 9. Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. Ciltacabtagene autoleucel, a B‐cell maturation antigen‐directed chimeric antigen receptor T‐cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE‐1): a phase 1b/2 open‐label study. Lancet North Am Ed. 2021;398(10297):314–324. [DOI] [PubMed] [Google Scholar]
- 10. Dogan A, Siegel D, Tran N, Fu A, Fowler J, Belani R, et al. B‐cell maturation antigen expression across hematologic cancers: a systematic literature review. Blood Cancer J. 2020;10(6):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khattar P, Pichardo J, Jungbluth A, Gao Q, Smith EL, Roshal M, et al. B‐cell maturation antigen is exclusively expressed in a wide range of B‐cell and plasma cell neoplasm and in a potential therapeutic target for BCMA directed therapies. Blood 2017;130:2755. [Google Scholar]
- 12. Moreau P, Garfall AL, van de Donk NWCJ, Nahi H, San‐Miguel JF, Oriol A, et al. Teclistamab in relapsed or refractory multiple myeloma. N Engl J Med. 2022;387(6):495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raghunandan S, Pauly M, Blum WG, Qayed M, Dhodapkar MV, Elkhalifa M, et al. BCMA CAR‐T induces complete and durable remission in refractory plasmablastic lymphoma. J Immunother Cancer. 2023;11(5):e006684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salem DA, Maric I, Yuan CM, Liewehr DJ, Venzon DJ, Kochenderfer J, et al. Quantification of B‐cell maturation antigen, a target for novel chimeric antigen receptor T‐cell therapy in Myeloma. Leuk Res. 2018;71:106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


