Abstract
Lumbar puncture (LP) is rarely complicated by cerebral vein thrombosis (CVT), especially if other risk factors coexist. We describe the case of a 28‐year‐old woman who developed CVT after corticosteroid treatment and LP performed for suspected multiple sclerosis. The day after LP, she developed intense headache and on Day 8 generalized tonic‐clonic seizures. A brain computed tomography scan showed thrombosis of the superior sagittal sinus and cortical veins. Thrombophilia screening showed heterozygous G20210A prothrombin mutation. Anticoagulant therapy with low molecular weight heparin and then warfarin was administered until Day 16 after LP, when a brain magnetic resonance imaging showed a subdural hematoma. Warfarin was interrupted and dabigatran was started. The patient recovered completely, both from the initial thrombotic event and the hemorrhagic complication. This case highlights the importance to keep in mind CVT in the differential diagnosis of post‐LP headache not responsive to standard therapy, and suggests that dabigatran can be considered an effective and safe treatment of CVT.
Keywords: anticoagulant therapy, cerebral vein thrombosis, direct oral anticoagulants, lumbar puncture, prothrombin mutation
1. INTRODUCTION
Cerebral vein thrombosis (CVT) is a rare manifestation of venous thrombosis, and accounts for a minor proportion of stroke events. The annual incidence ranges from 13.2 to 15.7 cases per million, and is more common in women than in men [1]. CVT is a rare complication of lumbar puncture (LP), together with other more common symptoms such as post‐dural puncture headache (PDPH) or transient nerve roots irritation. We report the case of a patient with a recent diagnosis of multiple sclerosis, who developed a CVT after a diagnostic LP concomitant to treatment with high‐dose corticosteroids.
2. CASE REPORT WITH RESULTS
A 28‐year‐old woman was admitted after onset of diplopia, blurred vision, and lower limbs hypoesthesia. Her past medical history was unremarkable. She was not taking drugs, including oral contraceptives, and was not pregnant. A brain contrast‐enhanced magnetic resonance imaging (MRI) showed multifocal lesions of the white matter suspected of multiple sclerosis. Treatment with high‐dose intravenous (IV) methylprednisolone (1 g daily for 5 days) was started, and an LP was performed. The day after, she developed an intense orthostatic headache associated with nausea and vomiting, which was managed with nonsteroidal anti‐inflammatory drugs and metoclopramide with a transient partial response. Neurological examination remained normal, and a brain computed tomography (CT) scan without contrast medium was normal. Hence, a brain contrast‐enhanced MRI was repeated, showing radiological signs of intracranial hypotension (diffuse linear pachymeningeal enhancement) without vascular abnormalities. The patient received levosulpiride and ketorolac with resolution of the headache. On Day 8 after LP, she developed two generalized tonic‐clonic seizures, successfully treated with diazepam and levetiracetam. A brain contrast‐enhanced CT scan showed thrombosis of the superior sagittal sinus and cortical veins at the vertex, which was confirmed by MRI (Figures 1 and 2). Anticoagulant therapy with enoxaparin (80 mg twice a day [BID], bodyweight 82 kg) was started, and after few days bridged with warfarin, maintaining the international normalized ratio (INR) between 2.0 and 3.0. Autoimmune and infectious diseases were ruled out, and a thrombophilia workup revealed a heterozygous G20210A prothrombin mutation. A brain MRI performed at Day 16 after LP showed a small subdural hematoma above the occluded cortical veins (the INR was within the therapeutic range). Warfarin was withdrawn, and enoxaparin at prophylactic doses was started. During the following days, the patient referred a gradual headache disappearance, and a brain CT scan without contrast medium performed at Day 22 after LP showed a complete reabsorption of the subdural hematoma. At this time, enoxaparin was substituted with dabigatran (150 mg BID). A brain contrast‐enhanced MRI performed 6 months later showed a partial recanalization of the CVT, which became complete after 12 months of therapy. Considering the complete recanalization of a provoked CVT, dabigatran was discontinued.
FIGURE 1.
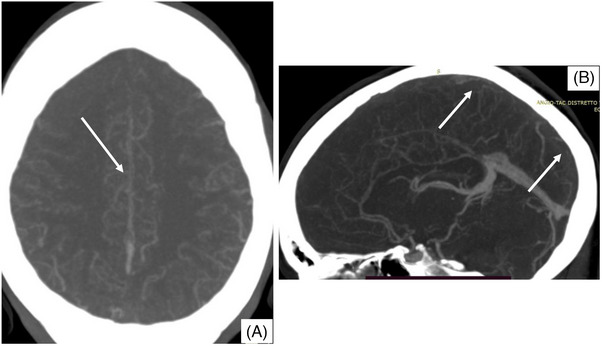
Computed tomography (CT) scan imaging showing thrombosis of the superior sagittal sinus. Contrast‐enhanced CT scan axial (panel A) and sagittal (panel B) sections showing thrombosis of the superior sagittal sinus.
FIGURE 2.
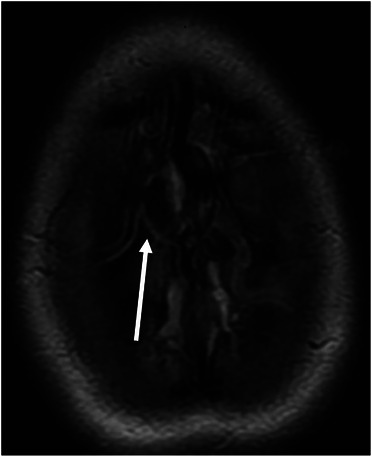
Magnetic resonance imaging showing thrombosis of the cortical veins at the vertex. Fluid‐attenuated inversion recovery (FLAIR) axial image showing hyperintensity of the cortical veins at the vertex draining in the superior sagittal sinus.
3. DISCUSSION
The coexistence of more than one predisposing factor is reported in 44% of patients with CVT [1]. Therefore, the search for thrombophilia abnormalities is suggested even when transient risk factors are present. The two most common gain‐of‐function mutations in coagulation factor V (G1691A, factor V Leiden) and prothrombin gene (G20210A) are associated with an increased risk of CVT, and the presence of a mutation (especially prothrombin G20210A) and oral contraceptive use further increase the risk. LP is usually a safe procedure, with serious side effects, including CVT, reported in less than 0.5% of cases [2]. However, an increasing number of case reports highlight LP as a risk factor for CVT and some pathogenetic mechanisms have been addressed to explain a causal relationship. First, in case of cerebrospinal fluid (CSF) loss, the intracranial volume is maintained through cerebral vascular dilatation. The consequent venous engorgement and reduction of venous blood flow velocity result in blood stasis and subsequent tendency to thrombosis. Second, intracranial hypotension leads to a negative spinal–cranial pressure gradient that can cause a stretching of the cerebral vessels and eventually a traumatic damage of the venous endothelium, which may also contribute to thrombosis. Third, CSF leakage leads to the reduction of its absorption into the cerebral venous sinuses, contributing to blood viscosity increase.
Most reported cases concern patients who develop CVT after receiving LP for demyelinating diseases [3], concomitantly with the administration of high‐dose IV corticosteroids. It is therefore suggested to use prophylactic doses of low molecular weight heparin after LP to prevent CVT in patients with suspected demyelinating diseases undergoing high‐dose IV corticosteroid treatment. Alternatively, if allowed, it would be cautious to reserve a window of at least 1 month between LP and high‐dose IV corticosteroids.
Early diagnosis of CVT is usually challenging, considering that symptoms may overlap with those of PDPH. We recommend suspecting CVT in all patients with PDPH that does not improve with the patient in decubitus, intensifies with time, or persists for more than 1 week. Moreover, de novo onset of bilateral papilledema associated with PDPH is consistent with a diagnosis of CVT.
Current guidelines do not recommend the use of direct oral anticoagulants (DOAC) for the treatment of CVT, despite some randomized controlled clinical trials reported comparable efficacy and a reduced incidence of intracranial bleeding compared to vitamin K antagonists (VKA) [4]. Based on this evidence, the decision to administer dabigatran after the development of a subdural hematoma during treatment with warfarin was made. Considering that approximately 40% of cases of CVT are complicated by intracranial hemorrhage, with subdural hematoma accounting for 11% of cases and superior sagittal sinus being associated with the higher bleeding risk [5], the decision to keep on going with anticoagulation in such patients is always challenging. In this scenario, this case report also shows the efficacy and safety of treatment with a DOAC in a patient with CVT who developed a subdural hematoma during treatment with VKA.
This case report highlights the importance to keep in mind CVT as a possible complication of LP, particularly when a patient develops worsening symptoms over time despite PDPH‐specific therapies. Albeit the incidence of intracranial hemorrhages in patients treated with DOAC seems lower than in those receiving VKA [4], controlled trials are needed to clarify the efficacy and safety of DOAC in the specific setting of patients with CVT.
AUTHOR CONTRIBUTIONS
All authors followed the patient, collected and analyzed the data, wrote the manuscript, and critically revised it.
CONFLICT OF INTEREST STATEMENT
The authors declare they have no conflicts of interest for this work.
FUNDING INFORMATION
There were no fundings for this work.
ETHICS STATEMENT
The authors have confirmed ethical approval statement is not needed for this submission.
CLINICAL TRIAL REGISTRATION
The authors have confirmed clinical trial registration is not needed for this submission.
PATIENT CONSENT STATEMENT
The authors have confirmed patient consent statement is not needed for this submission.
Marasco V, Gianniello F, Paolucci A, Martinelli I, Capecchi M. Post‐lumbar puncture cerebral vein thrombosis. eJHaem. 2024;5:222–224. 10.1002/jha2.803
DATA AVAILABILITY STATEMENT
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Capecchi M, Abbattista M, Martinelli I. Cerebral venous sinus thrombosis. J Thromb Haemost. 2018;16:1918–1931. 10.1111/jth.14210 [DOI] [PubMed] [Google Scholar]
- 2. Wilder‐Smith E, Kothbauer‐Margreiter I, Lämmle B, Sturzenegger M, Ozdoba C, Hauser SP. Dural puncture and activated protein C resistance: risk factors for cerebral venous sinus thrombosis. J Neurol Neurosurg Psychiatry. 1997;63:351–356. 10.1136/jnnp.63.3.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sillero Sánchez M, Rodriguez Fernandez N, Sánchez Vera L, Gómez González B, Asencio Marchante JJ. Cerebral venous thrombosis after lumbar puncture and treatment with high‐dose corticosteroids. Neurologia. 2014;29:315–316. 10.1016/j.nrl.2012.11.003 [DOI] [PubMed] [Google Scholar]
- 4. Wu T, Lv C, Wu L, Chen W, Lv M, Jiang S, et al. Risk of intracranial hemorrhage with direct oral anticoagulants: a systematic review and meta‐analysis of randomized controlled trials. J Neurol. 2022;269(2):664–675. 10.1007/s00415-021-10448-2 [DOI] [PubMed] [Google Scholar]
- 5. Afifi K, Bellanger G, Buyck PJ, Zuurbier SM, Esperon CG, Barboza MA, et al. Features of intracranial hemorrhage in cerebral venous thrombosis. J Neurol. 2020;267:3292–3298. 10.1007/s00415-020-10008-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


