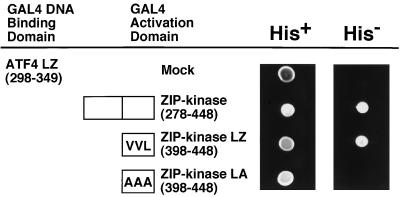
FIG. 5.
ZIP kinase and ATF4 interact via their leucine zippers. The C-terminal region (mouse ZIP kinase 278–448), the leucine zipper domain (ZIP kinase LZ), and the mutant with substitutions in the leucine zipper domain (ZIP kinase LA) were constructed in pACT2 to express the fusion protein with the GAL4 transactivation domain. Each construct was cotransformed into yeast Y190 cells with a plasmid expressing the leucine zipper domain of ATF4 fused to the GAL4 DNA binding domain. Growth of yeast on a synthetic dextrose agar plate lacking leucine, tryptophan, and histidine (His−) is indicative of a protein-protein interaction, while growth in the absence of leucine and tryptophan (His+) indicates a control.