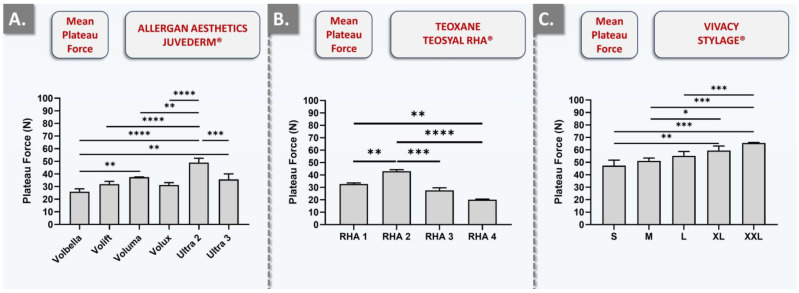
Figure 5.
Results of in vitro automated injectability studies for the assessment of dermal filler intra-brand injection force variability. The quantitative measurements were performed using the SimSkin® cutaneous equivalent and a constant plunger rod actuation speed of 1 mm·s−1. (A) Comparison of the mean plateau injection forces required for JUVÉDERM® products. (B) Comparison of the mean plateau injection forces required for TEOSYAL RHA® products. (C) Comparison of the mean plateau injection forces required for STYLAGE® products. Experiments were performed in triplicate, and the results were presented as average values assorted to corresponding standard deviations as error bars. Statistically significant differences (i.e., * or p-value < 0.05), very significant differences (i.e., ** or 0.001 < p-value < 0.01), or extremely significant differences (i.e., *** or 0.0001 < p-value < 0.001; **** or p-value < 0.0001) were found between the presented mean values. Detailed results of the statistical analyses are presented in Table S9.