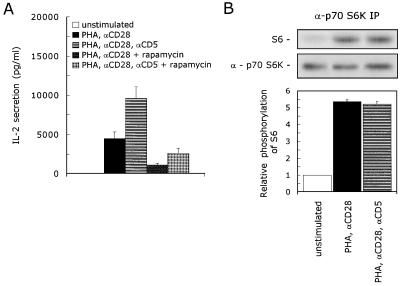
FIG. 4.
CD5 signaling is independent of p70 S6K activation. (A) T cells were stimulated with PHA with or without anti-CD28 (αCD28) and anti-CD5 (αCD5) in the presence or absence of 20 ng of rapamycin per ml. Cell-free supernatants were harvested after 24 h and analyzed for secreted IL-2 protein. The mean values ± SEM for the IL-2 secretion found in four independent experiments are shown. (B) T cells were left unstimulated or stimulated with PHA plus anti-CD28 (αCD28) in the presence or absence of anti-CD5 (αCD5) for 10 min. The cells were lysed, and immunoprecipitated p70 S6K was assayed for kinase activity with S6 peptide as a substrate. To ensure the equal precipitation of p70 S6K, the immunoprecipitates were loaded onto an SDS–12.5% polyacrylamide gel, and p70 S6K protein was detected by ECL Western blotting as described in Materials and Methods. The kinase assay shown is representative of two independent experiments. The specific p70 S6K kinase activity is determined by quantification of phosphorylated S6 with a PhosphorImaging system. The lower graph shows the relative phosphorylation of S6. The phosphorylation of S6 detected in unstimulated cells was set at 1. The mean values ± SEMs found in two independent experiments are shown.