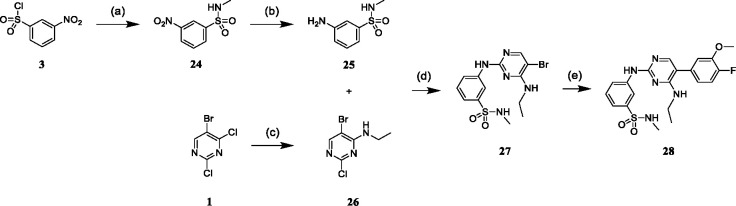
Scheme 2.
Synthesis of acyclic inhibitor 28.a
aReagents and conditions: (a) 2 M methylamine in tetrahydrofurane, triethylamine, tetrahydrofurane, 1 h, rt; (b) iron, ammonium chloride, methanol/water (9:1), reflux, 3 h; (c) 2 M ethylamine in tetrahydrofurane, triethylamine, tetrahydrofurane, 1 h, rt; (d) 4 N HCl in 1,4-dioxane, acetonitrile/water, reflux, 24 h; (e) potassium carbonate, [1,1’-Bis(diphenyl-phosphino)ferrocene]-palladium(II) dichloride, 4-fluoro-3-methoxyphenylboronic acid, 1,4-dioxane/dimethylformamide (1:1), 100 °C, μW, 2 h.