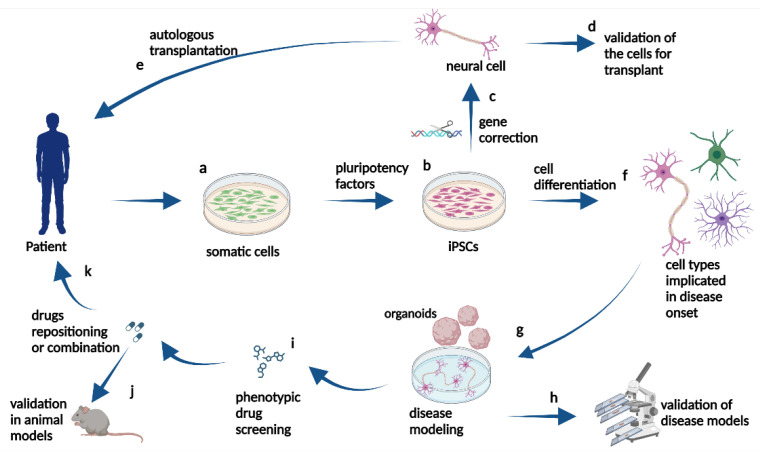
Figure 1.
Schematic representation of iPSC applications. Somatic cells (a) can be obtained from patients with neurodegenerative diseases and induced to differentiate into iPSCs by pluripotency factors (b). Then, they can be genetically manipulated to undergo gene correction (c) and differentiated (f) into cell types implicated in disease onset. iPSCs can be differentiated into healthy neural cells for correct cell function and validation for transplant (d) or autologous transplantation in donor patients (e). Alternatively, genetically corrected iPSCs (b) can be differentiated into neural cells implicated in disease onset (f) to model the cellular pathogenic phenotype in vitro (g). These cellular cultures or organoids can be studied in the laboratory to validate iPSC-derived models (h) and can be used to screen drugs, for example (i). The selected drugs can be tested in animal models in preclinical trials (j), and in the case of beneficial results indicating drug repositioning or combination therapy (k), clinical trials in patients can be proposed.