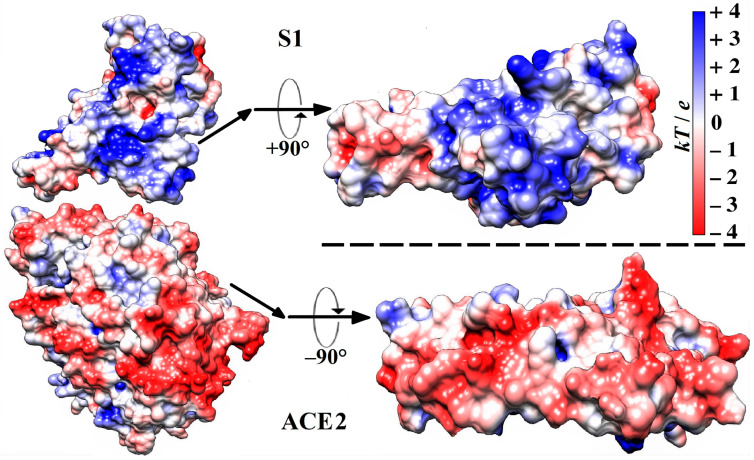
Figure 1.
Molecule models (all C, O, N, and H atoms are included) of a section of the S1-ACE2 complex (according to its crystallographic structure deposited in Protein Data Bank: 6LZG) in the region of the receptor-binding domain (RBD) (on the left), and the contact surfaces (on the right) by which the human ACE2 receptor associates with the S1-subunit of the wild-type strain of SARS-CoV-2 β-coronavirus. The two protein globules on the right are rotated by −90° (S1) and +90° (ACE2) to display the receptor-binding and the virus-binding contacting surfaces, respectively. The electrostatic potential φ = kT/e on the surfaces of the two globular proteins is computed using their atomic coordinates and visualized by coloration according to its sign and value in blue (positive), red (negative), and white (neutral) with scale kT/e = ±4 J/C (k—Boltzmann constant [J/K], T—absolute temperature [K], e—charge of the proton [C]; 1 kT/e [J/C] = 26.7 mV at 37 °C).