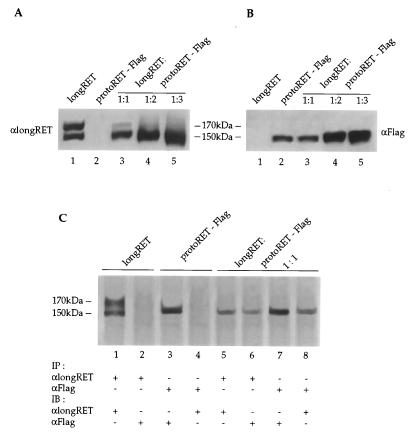
FIG. 9.
LongRET and the Flag mutant are coexpressed and form heterodimers in cotransfected cells. (A and B) Cos cells were cotransfected with 5 μg of longRET and 5, 10, and 15 μg of protoRET-Flag expression vectors. Parallel transfections were carried out with 5 μg of longRET and protoRET-Flag expression vectors alone. A Western blot analysis was performed with increasing amounts of proteins from the transfected cells. Lane 1, longRET (50 μg); lane 2, protoRET-Flag (50 μg); lanes 3 to 5, protoRET and protoRET-Flag at a 1:1 ratio (100 μg) (lane 3), at a 1:2 ratio (150 μg) (lane 4), and at a 1:3 ratio (200 μg) (lane 5). After the immunoblotting, the RET species were stained with either anti-longRET- or anti-Flag-specific antibodies, as indicated. The migration of the 170- and 150-kDa RET monomers is shown. (C) Immunoprecipitation and immunoblotting experiments. One milligram of protein extracts from Cos cells transfected with longRET and protoRET-Flag expression vectors alone or cotransfected at a 1:1 molar ratio was subjected to immunoprecipitation with anti-longRET (αlongRET) (lanes 1, 2, 5, and 6) or anti-Flag (αFlag) antibodies (lanes 3, 4, 7, and 8). The anti-longRET immunocomplexes were subsequently stained with the same antibodies (lanes 1 and 5) or with the anti-Flag antibodies (lanes 2 and 6). The anti-Flag immunoprecipitates were stained with the same antibodies (lanes 3 and 7) or with anti-longRET antibodies (lanes 4 and 8). The migration of the 170- and 150-kDa RET monomers is shown.