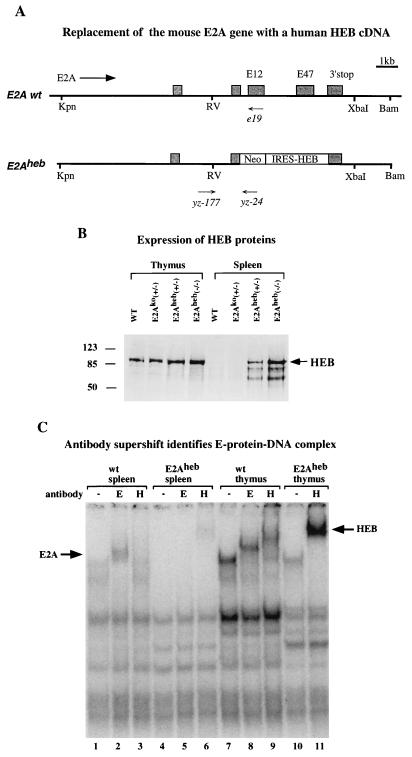
FIG. 5.
(A) Gene targeting construct for generating the E2Aheb allele. The Neo marker was introduced in the E2A gene as a fusion protein. IRES-driven human HEB cDNA was inserted downstream of the Neo cassette but in front of the E2A translation termination site. Both E12 and E47 exons are completely deleted from the targeting construct. Primers used for PCR genotyping the mutant and wild-type (wt) alleles are indicated (see Materials and Methods). Designations are as in Fig. 1A. (B) Western blot analysis of thymus (lanes 1 to 4) and spleen (lanes 5 to 8) nuclear extracts from 10-day-old neonatal mice of indicated genotypes. Wild-type, E2Ako heterozygote, E2Aheb heterozygote, and E2Aheb homozygote samples are included in the assay as indicated. The anti-HEB polyclonal sera used in this assay (Santa Cruz) cross-react with both human and mouse HEB proteins. Size markers in kilodaltons and the HEB band are indicated on the left and right, respectively. (C) Gel shift analysis of E-protein complex formation, using μE5 radiolabeled probe. μE5 probe (25) was incubated with nuclear extracts from splenocytes (lanes 1 to 6) or thymocytes (lanes 7 to 11) derived from wild-type (lanes 1, 2, 3, 7, 8, and 9) or E2Aheb (lanes 4, 5, 6, 10, and 11) animals. Anti-E2A antibody (Yae) was added in lanes 2, 5, and 8, and anti-HEB antibody (Santa Cruz) was added in lanes 3, 6, 9, and 11. The E2A- and HEB-dependent supershifts are indicated by arrows on the left and right sides, respectively. Assay conditions are as described by Sawada and Littman (22).