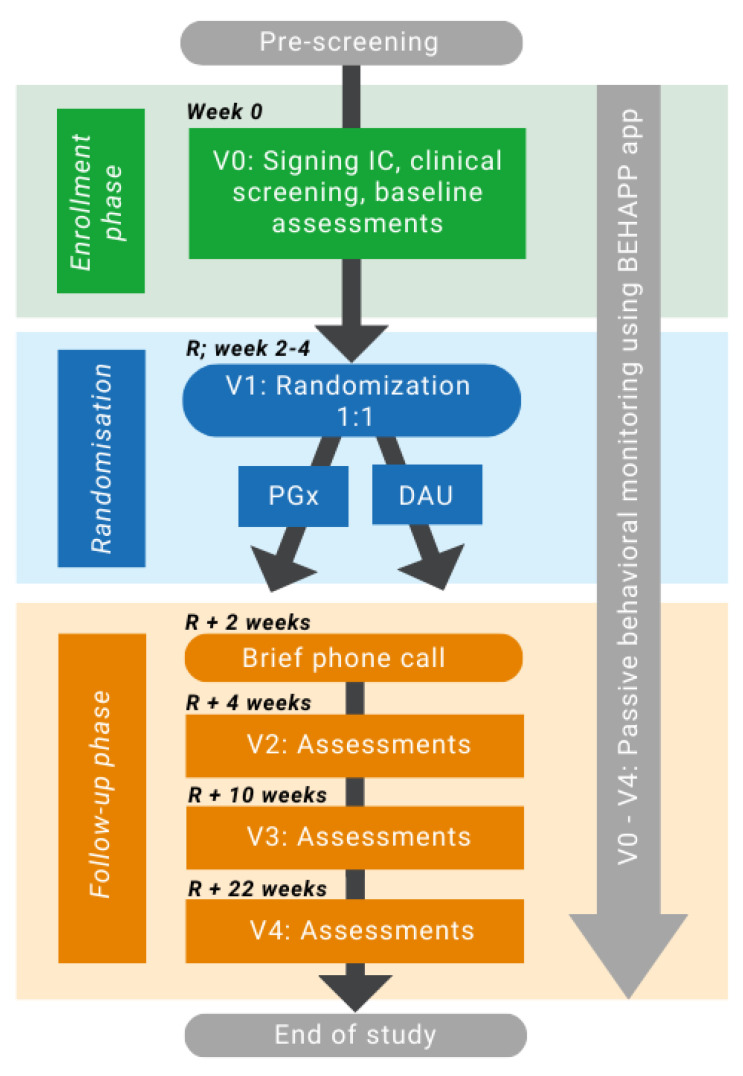
Figure 1.
Study flow chart. The study period comprises 24–26 weeks and includes a baseline visit, a randomization visit, three follow-up visits, and a phone call. Behavioral monitoring using the Behapp application [54] will be conducted during the whole study period. Baseline visit (V0) and Visit two assessments include somatic measurements (ECG, blood sampling, weight, blood pressure, heart rate), clinical modulators, and questionnaires. Visit 3 (V3) and 4 (V4) assessments include clinical modulators, anthropometric measurements (weight, blood pressure, heart rate), and psychometric questionnaires. Abbreviations: IC = informed consent; DAU = dosing as a usual group; PGx = pharmacogenetic-guided group; R = randomization; V = visit.