Table 2.
Substrate scope of acylhydrazides a.
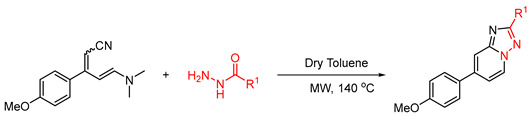
| Entry No | Compound Code | R1 | Time (h) | Yield (%) | Under Reflux Conditions b |
|---|---|---|---|---|---|
| 1 | 3a | phenyl | 3.5 | 83 | 8 h, 85% |
| 2 | 3b | 4-methoxyphenyl | 3 | 89 | 6 h, 89% |
| 3 | 3c | 4-tolyl | 3 | 82 | |
| 4 | 3d | 4-(trifluoromethyl)phenyl | 3 | 74 | |
| 5 | 3e | 4-nitrophenyl | 3.5 | 24 | 8 h, 24% |
| 6 | 3f | 4-chlorophenyl | 3 | 41 | 6 h, 42% |
| 7 | 3g | 4-bromophenyl | 3 | 43 | |
| 8 | 3h | 3-pyridinyl | 3 | 77 | |
| 9 | 3i | 2-thiophenyl | 2 | 94 | 5 h, 85% |
| 10 | 3j | 2-furanyl | 2 | 73 | |
| 11 | 3k | methyl | 7 | 67 | 12 h, 66% |
| 12 | 3l | heptyl | 6 | 46 |
a Reaction conditions: 4-methoxy enaminonitrile (0.2 mmol, 1.0 equiv.), acyl hydrazides (0.40 mmol, 2.0 equiv.), dry toluene (1.5 mL), microwave heating at 140 °C for the indicated time. b The reaction was performed under reflux conditions stirred at 120 °C, along with 100 g of 3Å MS in 2.0 mL dry toluene. Isolated yields.
