Abstract
Age-related tau astrogliopathy (ARTAG) is detectable in the brains of over one-third of autopsied persons beyond age 80, but the pathoetiology of ARTAG is poorly understood. Insights can be gained by analyzing risk factors and comorbid pathologies. Here we addressed the question of which prevalent co-pathologies are observed with increased frequency in brains with ARTAG. The study sample was the National Alzheimer's Coordinating Center (NACC) data set, derived from multiple Alzheimer’s disease research centers (ADRCs) in the United States. Data from persons with unusual conditions (e.g. frontotemporal dementia) were excluded leaving 504 individual autopsied research participants, clustering from 20 different ADRCs, autopsied since 2020; ARTAG was reported in 222 (44.0%) of included participants. As has been shown previously, ARTAG was increasingly frequent with older age and in males. The presence and severity of other common subtypes of pathology that were previously linked to dementia were analyzed, stratifying for the presence of ARTAG. In logistical regression-based statistical models that included age and sex as covariates, ARTAG was relatively more likely to be found in brains with limbic-predominant age-related TDP-43 encephalopathy neuropathologic change (LATE-NC), and in brains with comorbid cerebrovascular pathology (arteriolosclerosis and/or brain infarcts). However, ARTAG was not associated with severe Alzheimer’s disease neuropathologic change (ADNC), or primary age-related tauopathy (PART). In a subset analysis of 167 participants with neurocognitive testing data, there was a marginal trend for ARTAG pathology to be associated with cognitive impairment as assessed with MMSE scores (P=0.07, adjusting for age, sex, interval between final clinic visit and death, and ADNC severity). A limitation of the study was that there were missing data about ARTAG pathologies, with incomplete operationalization of ARTAG according to anatomic region and pathologic subtypes (e.g., thorn-shaped or granular-fuzzy astrocytes). In summary, ARTAG was not associated with ADNC, whereas prior observations about ARTAG occurring with increased frequency in aging, males, and brains with LATE-NC were replicated. It remains to be determined whether the increased frequency of ARTAG in brains with comorbid cerebrovascular pathology is related to local infarctions or neuroinflammatory signaling, or with some other set of correlated factors including blood-brain barrier dysfunction.
Keywords: Astrocytes, MAPT, Aging, Tauopathy, Amygdala, Small vessel disease, Stroke, Infarction, VCID, Thorn-shaped astrocytes
1. Introduction
Age-related tau astrogliopathy (ARTAG) is a common pathologic feature of brain aging (Di et al., 2023; Iida et al., 2021; Kovacs et al., 2016; Kovacs et al., 2017c; Resende et al., 2020; Yagita et al., 2022). The diagnostic hallmark of ARTAG is phosphorylated tau protein (pTau)-immunoreactive inclusions within astrocytes (Kovacs et al., 2016; Kovacs et al., 2017c). Tau-immunoreactive astrocytes in ARTAG may display a range of histomorphologic appearances, including so-called thorn-shaped astrocytes in subpial, subependymal, and white matter regions (Ikeda et al., 1998; Ikeda et al., 1995; Schultz et al., 2004). Other subtypes of ARTAG include “granular-fuzzy astrocytes” and astrocytic pTau aggregates that can be seen in gray matter (Kovacs et al., 2016).
Much remains to be learned about ARTAG, including the clinical-pathological correlations (Iida et al., 2021; Robinson et al., 2018). No clinical biomarker is currently known to reliably identify individuals with ARTAG during life and the pathogenetic mechanism(s) that underlie ARTAG are largely unknown. In the present study, we focused on the pathological correlates of ARTAG.
Pathologic lesions in aging brains often occur in combinations—pathologically “pure” (only a single lesion-type represented) patterns of brain pathology are relatively unusual in persons beyond age 80(Jellinger, 2022; Kapasi et al., 2017; Kovacs et al., 2013; McAleese et al., 2016). Different pathologic comorbidities may either synergize with each other (one pathology exacerbating the other) or else may be caused by common “upstream” factors wherein both pathologies may result from shared original cause(s), such as genetic or environmental factors (Chornenkyy et al., 2019). On the other hand, some common mixed pathologies can be observed in the same brain coincidentally after having developed independently. Teasing out the various complex pathoetiological mechanisms requires a broad repertoire of experimental systems, but primarily, relevant insights must be gathered from autopsy cohort studies to analyze clues about how the different lesion subtypes correlate with each other in the aging human brain.
A specific context where astrocytic pTau pathology can be seen is in brains affected by traumatic brain injury (TBI) and/or chronic traumatic encephalopathy (CTE) (Bachstetter et al., 2021; Bieniek et al., 2021; Forrest et al., 2019; McKee et al., 2009). This establishes that environmental influences (specifically mechanical trauma) can cause ARTAG-like pathological phenomena. However, the main pathological hallmark of TBI/CTE appears to be intraneuronal, rather than intra-glial pTau lesions(Butler et al., 2022).
Open questions remain about other pathological correlates between ARTAG and common neurodegenerative pathologies, e.g., tauopathies such as Alzheimer’s disease neuropathologic change (ADNC) (Montine et al., 2012) and primary age-related tauopathy (PART) (Crary et al., 2014). Other common proteinopathies include Lewy body diseases (LBD) (Attems et al., 2021) and limbic-predominant age-related TDP-43 encephalopathy neuropathologic changes (LATE-NC) (Nelson et al., 2019). Indeed, a recent study on 101 individuals from a European ageing community-dwelling cohort demonstrated an association of LATE-NC with the presence of subpial, subependymal, and perivascular ARTAG (Forrest et al., 2022). Importantly, cognitive decline is not only linked to neurodegenerative proteinopathies. There are also multiple subtypes of cerebral vascular pathologies (Skrobot et al., 2016), which reflect ischemia, metabolic insufficiency, hemorrhage, and/or neuroinflammation, and can have a strong influence on neurological function. Whereas many of these pathologies have been shown to have synergistic influence on each other, and/or common upstream risk factors, their dispositions with regard to the presence or absence of ARTAG are incompletely characterized to date.
Here we analyzed data relating to pathologic correlates of ARTAG in the National Alzheimer's Coordinating Center (NACC) data set. This publicly available data storage and dissemination resource includes anonymized information that has been aggregated from the US National Institute on Aging–funded Alzheimer's Disease Research Centers (ADRCs). Contributory ADRCs vary in their recruitment practices and assessment protocols while providing standardized assessment instruments. In the participants with any ARTAG workup data available, the presence or absence of ARTAG was tested for associations with the presence and severity of other subtypes of pathology that were previously linked to dementia. Factoring in relevant covariates such as sex and age at death, we found that ARTAG was positively associated with LATE-NC and cerebrovascular pathologies, but not with the other common age-related tauopathies, ADNC or PART.
2. Methods
2.1. Study participants
Participants were assessed using the standardized NACC Uniform Data Set (UDS) (Beekly et al., 2007; Besser et al., 2018a) at their local ADRC approximately annually, including participant demographics, health history, physical and neurological exams, and AD and related dementias symptomology. Standardized data collected on neuropathological features present at the time of death are available for participants who were assessed with the UDS and who consented to autopsy (Besser et al., 2018b; Mock et al., 2020). Participants who met the study’s eligibility criteria were selected from the June 2023 (NACC62) data freeze. These were restricted to participants who died and underwent autopsy since 2020 (when ARTAG data collection was started in the NACC dataset). In the analyzed sample, we excluded participants who had at least one rare disease (e.g., frontotemporal lobar degeneration [FTLD], multiple system atrophy [MSA], and others) shown in Supplementary Table 1 and included those with autopsy data available. We further limited to ADRCs with ≥2 eligible participants with autopsy confirmed (and reported to NACC) ARTAG (Supplementary Table 2). Because the focal point of the current study was on common aging-related conditions, we limited the sample to individuals beyond age 60 at death.
2.2. Neuropathology data
ARTAG data were provided from a subset of the ADRCs after that parameter was included starting in 2020 (NACC Neuropathology (NP) Data Set v11). The ARTAG related variables includes the presence ARTAG with 0 = no and 1 = yes (the variable name in NACC NP v10 dictionary is NPARTAG), severity of ARTAG with 1 = mild, 2 = moderate, and 3 = severe (NPATGSEV), and anatomical localizations of ARTAG in amygdala and/or frontal neocortex (NPATGAMY with 0 = no and 1 = yes and NPATGFRN with 0 = no and 1 = yes). Specifically subtyped (i.e., subpial, subependymal, perivascular, white and gray matter) ARTAG neuropathology data are only from the amygdala and frontal cortex in this data set. Tau NFTs was operationalized according to Braak NFT stages (Braak and Braak, 1991) and categorized into four (Stage 0, Stage I or II, Stage III or IV, and Stage V or VI). Regional progression of amyloid plaques was represented by four categorized Thal Phase ratings (Phase 0, Phase 1-2, Phase 3, and Phase 4-5) (Thal et al., 2002). Density of neocortical neuritic plaques were based on Consortium to Establish a Registry for Alzheimer's Disease (CERAD) ratings (none, sparse, moderate, and frequent) (Mirra, 1997; Mirra et al., 1991). TDP-43 neuropathologies were specified in three brain regions: amygdala, entorhinal/inferior temporal cortex and/or hippocampus, and neocortex. Lewy body pathology (LBP) data were dichotomous with 0 = none and 1 = present. For cerebrovascular pathologies, data were available on cerebral amyloid angiopathy (NACCAMY) (none, mild, moderate, and severe), arteriolosclerosis (NACCARTE) (none, mild, moderate, and severe), infarcts and lacunes (NPINF) (no and yes), microinfarcts (NACCMICR) (no and yes).
2.3. Associations between ARTAG and cerebrovascular/cardiovascular risk factors
Diabetes was defined as 0 = no and 1 = yes using any history of diabetes from the participants' health history and the clinician-assessed medical conditions forms (DIABETES and DIABET) and a history of antidiabetic medication (NACCDBMD) at the last NACC UDS visit. Hypertension and hypercholesterolemia conditions were determined in the similar way with diabetes using five variables for hypertension (HYPERTEN, HXHYPER, HYPERT, NACCAHTN, and NACCHTNC) and three variables for hypercholesterolemia (HYPCHOL, HYPCHOL, and NACCLIPL), respectively. Any history of heart attack/cardiac arrest (CVHATT, 0 = no and 1 = yes) was measured in the subject health history and congestive heart failure (CONGHRT, 0 = no and 1 = yes) present was measured in the clinician-assessed medical conditions form.
2.4. Cognitive tests
Cognitive test scores at the last visit were drawn from the NACC Uniform Data Set (UDS) (Weintraub et al., 2009), including Mini Mental State Examination (MMSE) (Folstein et al., 1975), Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005), Animal and Vegetable Naming (Morris et al., 1989), Wechsler Memory Scale-Revised (WMS-R) Logical Memory – immediate and delayed (Wechsler, 1987), Craft Story 21 Recall – immediate and delayed (Craft et al., 1996), and Digit span forward and backward trials(Richardson, 2007). Since the MoCA, Craft Story 21 Recall – immediate and delayed, and Number Span Test: Forward and Backward were introduced in the NACC UDS version 3 from March 2015, replacing MMSE, WMS-R Logical Memory – immediate and delayed, and Digit span forward and backward trials, we transformed the new battery scores into equivalent old battery scores based on Monsell and colleagues’ crosswalk study (Monsell et al., 2016). We included participants who had these cognitive test scores at the last visit measured within 6 years before death.
2.5. Statistical analysis
All statistical analyses were conducted in R version 4.2.1(R Core Team, 2021). We estimated odds ratio (OR) and its 95% confidence interval (CI) to examine the associations of NP and CVD risk factors with the ARTAG binary outcomes (i.e., NPARTAG with 0 = no and 1 = yes) using a logistic regression with covariates. We ran two logistic regression models with different set of the covariates: (1) adjusted for age at death and sex (Model 1); (2) additionally adjusted for the number of APOE ε4 alleles and Braak NFT stage with four response categories (Model 2). The second model was built to remove ADNC effects. For examining the association between cognitive test scores and ARTAG, we also included time-interval between the last visit and death as a covariate in both the models.
3. Results
After exclusions were applied, the final analytic sample included 504 participants sourced from 20 ADRC autopsy cohorts. The mean age at death was 82.7 years (standard deviation (SD) = 9.9), the mean of years in education was 16.7 (SD = 7.9), and 53.4% were females. 36.2% had one ε4 allele and 9.2% had two ε4 alleles (Table 1). More than half of the participants had severe ADNC (Braak NFT stages V or VI) and ~90% had any Aβ plaques, ~80% Thal Aβ phase 3 or higher (Table 2). Approximately one-third of included participants (~35%) had TDP-43 pathology in amygdala, hippocampus, and/or entorhinal cortex (Table 2).
Table 1.
Demographic and APOE alleles of included subjects
| Characteristics | n = 504 |
|---|---|
| Age at death, mean ± SD | 82.7 ± 9.9 |
| Years in education, mean ± SD | 16.7 ± 7.9 |
| Sex, n (%) | |
| Male | 235 (46.6) |
| Female | 269 (53.4) |
| APOE genotype, n (%) | |
| −/− | 251 (54.7) |
| −/ε4 | 166 (36.2) |
| ε4/ε4 | 42 (9.2) |
Table 2.
Neuropathologic features of included subjects
| Characteristics | Overall (n = 504) |
ARTAG | P-value* | ||
|---|---|---|---|---|---|
| No (n = 282) |
Yes (n = 222) |
||||
| Braak NFT stage, n (%) | |||||
| 0 | 12 (2.4) | 9 (3.2) | 3 (1.4) | 0.045 | |
| I-II | 84 (16.8) | 48 (17.1) | 36 (16.3) | ||
| III-IV | 109 (21.8) | 49 (17.5) | 60 (27.1) | ||
| V-VI | 296 (59.1) | 174 (62.1) | 122 (55.2) | ||
| Thal phase, n (%) | |||||
| 0 | 48 (9.5) | 26 (9.2) | 22 (9.9) | 0.54 | |
| 1-2 | 51 (10.1) | 25 (8.9) | 26 (11.7) | ||
| 3 | 51 (10.1) | 26 (9.2) | 25 (11.3) | ||
| 4-5 | 354 (70.2) | 205 (72.7) | 149 (67.1) | ||
| Neuritic plaques, n (%) | |||||
| No | 111 (22.0) | 61 (21.6) | 50 (22.5) | 0.72 | |
| Sparse | 56 (11.1) | 29 (10.3) | 27 (12.2) | ||
| Moderate | 101 (20.1) | 54 (19.1) | 47 (21.2) | ||
| Frequent | 236 (46.8) | 138 (48.9 | 98 (44.1) | ||
| TDP-43 in amygdala, n (%) | |||||
| No | 304 (64.0) | 192 (73) | 112 (52.8) | 6.0 × 10−6 | |
| Yes | 171 (36.0) | 71 (27) | 100 (47.2) | ||
| TDP-43 in hippocampus/EC, n (%) | |||||
| No | 237 (62.4) | 134 (68.7) | 103 (55.7) | 0.011 | |
| Yes | 143 (37.6) | 61 (31.3) | 82 (44.3) | ||
| TDP-43 in neocortex, n (%) | |||||
| No | 390 (94.0) | 211 (97.2) | 179 (90.4) | 0.0037 | |
| Yes | 25 (6.0) | 6 (2.8) | 19 (9.6) | ||
| Lewy bodies, n (%) | |||||
| No | 295 (58.6) | 163 (57.8) | 132 (59.7) | 0.72 | |
| Yes | 208 (41.4) | 119 (42.2) | 89 (40.3) | ||
| Cerebral amyloid angiopathy, n (%) | |||||
| None | 148 (29.4) | 78 (27.7) | 70 (31.5) | 0.28 | |
| Mild | 171 (33.9) | 106 (37.6) | 65 (29.3) | ||
| Moderate | 124 (24.6) | 66 (23.4) | 58 (26.1) | ||
| Severe | 61 (12.1) | 32 (11.3) | 29 (13.1) | ||
| Arteriolosclerosis, n (%) | |||||
| None | 68 (13.5) | 47 (16.7) | 21 (9.5) | 0.0027 | |
| Mild | 201 (40.0) | 123 (43.8) | 78 (35.1) | ||
| Moderate | 163 (32.4) | 79 (28.1) | 84 (37.8) | ||
| Severe | 71 (14.1) | 32 (11.4) | 39 (17.6) | ||
| Infarcts and lacunes, n (%) | |||||
| No | 422 (84.1) | 250 (89) | 172 (77.8) | 8.7 × 10−4 | |
| Yes | 80 (15.9) | 31 (11) | 49 (22.2) | ||
| Microinfarcts, n (%) | |||||
| No | 398 (79.0) | 32 (82.3) | 166 (74.8) | 0.047 | |
| Yes | 106 (21.0) | 50 (17.7) | 56 (25.2) | ||
P-value is calculated by Fisher’s exact test
SD = standard deviation; NFT = neurofibrillary tangles
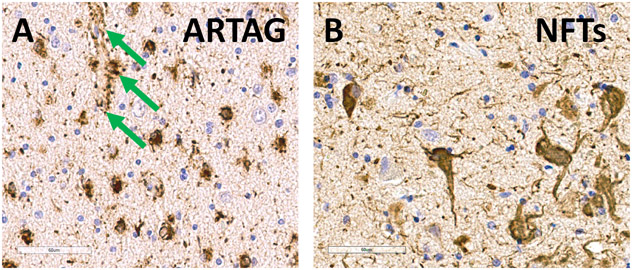
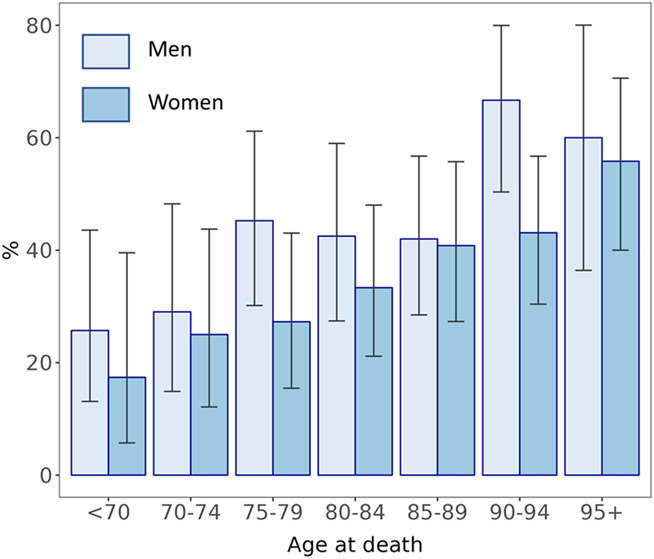
The focus of the study was on ARTAG – astroglial pTau pathology – of which photomicrographs are depicted in Figure 1; any detected positive ARTAG in the brain was considered to be a positive case (the subtypes are described in Supplemental Table 3). Among included participants, 222 (44.0%) had autopsy-confirmed ARTAG (NPARTAG=1). These cases were derived from 20 different ADRCs (Supplementary Table 2). The median number of ARTAG cases per ADRC was 9.5; range 2-28 ARTAG cases per ADRC. The median number of controls per ADRC was 10.5; range 1-28 non-ARTAG controls per ADRC. There were trends for ARTAG to become more common in advanced age, and it was relatively frequently observed in males (Figure 2). For all subsequent analyses, we ran separate logistic regression models, factoring in at least age and sex as covariates, for each of the pathologies as an independent variable.
Figure 1. Photomicrographs depict age-related astrogliopathy (ARTAG; A) in comparison with Alzheimer’s/PART-type neurofibrillary tangles (NFTs; B) in the same brain, stained immunohistochemically for phosphorylated tau (pTau) labeled brown.
The tissue was counterstained with hematoxylin (blue, highlighting cell nuclei). Panel A shows pTau within compact white matter thorn-shaped astrocytes in the amygdala region. In the present study, the location most noted to harbor ARTAG was the amygdala. Highlighted with green arrows is ARTAG pathology surrounding a small blood vessel. By contrast, Panel B shows larger, intraneuronal NFTs in the entorhinal cortex. Scale bars = 60microns in both panels. The pTau antibody used here was the monoclonal PHF-1 (1:500 dilution), a gift from Dr. Peter Davies.
Figure 2. Percent of brains with ARTAG, stratifying by age and sex, in the NACC Data set.
Pale bars represent men and darker blue bars represent women. Shown are results from the NACC Data set among autopsied subjects with ARTAG status known (n=504), sourced from 20 different independent United States Alzheimer’s Disease Research Centers. In this sample there were two general trends: ARTAG was more frequent with aging, and in males. These findings are compatible with prior results from a separate sample of research volunteers (Kovacs et al., 2018a). Error bars = stdev.
Table 3 shows that the associations of the presence or absence of ARTAG with other neuropathologies, adjusted for age at death and sex (these covariates were necessary as shown in the data in Figure 2). TDP-43 pathology observed in amygdala and neocortex were significantly associated with ARTAG when adjusting for age at death and sex (OR >2 for each). Relative to LATE-NC Stage 0 (no detected TDP-43 pathology), LATE-NC stages 1, 2, and 3 each showed association with the presence of ARTAG. By contrast, the same model did not detect associations between ARTAG and ADNC or LBD pathologies.
Table 3.
Associations between ARTAG (NPARTAG = 0 or 1) and other neurodegenerative protein pathologies
| Neuropathology | ||
|---|---|---|
| OR* | 95% CI* | |
| Braak NFT stage* | ||
| 0 | Reference | |
| I-II | 1.88 | 0.50 – 9.16 |
| III-IV | 2.58 | 0.69 – 12.45 |
| V-VI | 2.15 | 0.61 – 10.05 |
| Thal phase | ||
| 0 | Reference | |
| 1-2 | 1.17 | 0.52 – 2.65 |
| 3 | 1.16 | 0.51 – 2.64 |
| 4-5 | 0.99 | 0.53 – 1.87 |
| Neuritic plaques | ||
| No | Reference | |
| Sparse | 0.92 | 0.47 – 1.80 |
| Moderate | 1.04 | 0.60 – 1.83 |
| Frequent | 1.20 | 0.74 – 1.96 |
| TDP-43 in amygdala | ||
| No | Reference | |
| Yes | 2.05 | 1.37 – 3.07 |
| TDP-43 in hippocampus/EC | ||
| No | Reference | |
| Yes | 1.54 | 0.99 – 2.41 |
| TDP-43 in neocortex | ||
| No | Reference | |
| Yes | 3.22 | 1.29 – 9.23 |
| LATE-NC stage | ||
| 0 | Reference | |
| 1 | 3.12 | 1.50 – 6.81 |
| 2 | 1.81 | 1.13 – 2.91 |
| 3 | 4.60 | 1.82 – 13.26 |
| Lewy bodies | ||
| No | Reference | |
| Yes | 1.04 | 0.72 – 1.51 |
LATE-NC = limbic-predominant age-related TDP-43 encephalopathy neuropathologic change; OR=Odds ratio; CI=Confidence interval; *The OR and CI are adjusted for age at death and sex
We also observed the significant associations between cerebrovascular pathologies and ARTAG (Table 4). Here we included a model that factored in ADNC because there have been shown to be synergies between ADNC and cerebrovascular pathologies—thus Model 1 adjusted for age at death and sex whereas Model 2 additionally adjusted for the number of the APOE ε4 alleles and three categorized (“B”) Braak NFT stages. The significant association with infarcts and lacunes were robust (OR = 1.83 and 95% CI = 1.10 – 3.06 in Model 1 and OR = 2.19 and 95% CI = 1.28 – 3.82 in Model 2).
Table 4.
Associations between ARTAG (NPARTAG = 0 or 1) and cerebrovascular pathologies
| Neuropathology | Model 1 | Model 2 | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Cerebral amyloid angiopathy | ||||
| None | Reference | |||
| Mild | 0.81 | 0.51 – 1.30 | 0.83 | 0.49 – 1.42 |
| Moderate | 1.18 | 0.72 – 1.95 | 1.13 | 0.63 – 2.04 |
| Severe | 1.09 | 0.59 – 2.03 | 0.86 | 0.42 – 1.76 |
| Arteriolosclerosis | ||||
| None | Reference | |||
| Mild | 1.34 | 0.74 – 2.48 | 1.28 | 0.69 – 2.43 |
| Moderate | 2.01 | 1.09 – 3.78 | 2.16 | 1.13 – 4.20 |
| Severe | 2.34 | 1.15 – 4.85 | 2.12 | 0.99 – 4.61 |
| Infarcts and lacunes | ||||
| No | Reference | |||
| Yes | 1.83 | 1.10 – 3.06 | 2.19 | 1.28 – 3.82 |
| Microinfarcts | ||||
| No | Reference | |||
| Yes | 1.26 | 0.81 – 1.98 | 1.39 | 0.86 – 2.25 |
Model 1 is with the adjustment of age at death and sex, and Model 2 is with the additional adjustment of the number of the ε4 alleles in APOE and four categorized Braak NFT stage.
Since ARTAG and ADNC stand as separate from PART, and the three together constitute the major three subtypes of aging-related tauopathy, we performed a separate analysis of the association between PART severity (Braak stages I/II vs III/IV in cases lacking amyloid plaques) and ARTAG. In the participants who had probable PART, Braak NFT scores as operationalized above were not significantly associated with ARTAG (Table 5).
Table 5.
Participants who had Probable PART (CERAD neuritic plaque score = None) and Braak = 0 to IV (n = 107)
| Braak NFT stage* | ||
|---|---|---|
| OR | 95% CI | |
| 0-II | Reference | |
| III-IV | 1.72 | 0.66 – 4.52 |
CI = Confidence interval; Regression model factors in covariates age of death and sex.
Although the main focus of the present study was on pathological (ARTAG)-pathological comorbidity associations, we also performed additional association tests between clinical parameters with eventual ARTAG diagnosis at autopsy. We first evaluated the associations between cardiovascular risk factors with ARTAG, pursuant to the findings of positive associations between cerebrovascular pathology and ARTAG (Table 4). We did not find any significant associations of CVD risk factors with ARTAG (Table 6). Next, we tested the results of neurocognitive testing and eventual ARTAG diagnoses among the 441 participants who had a final clinic visit within 6 years between the last follow-up visit and death. Again, because ADNC has a strong impact on cognition, we included a model that adjusted only for age at death, sex, and the interval between the last clinical visit and death (Model 1) and a separate model that also adjusted for the number of the APOE ε4 alleles and Braak NFT stages (Model 2). These results are shown in Table 7, with the caveat that approximately 2/3rd of the participants in the total sample lacked neurocognitive testing (sample sizes for each test are shown in Table 7). Factoring in the sample size consideration, there was a possible nominal trend for MMSE scores to be lower in those with ARTAG (P<0.1).
Table 6.
Associations between ARTAG (NPARTAG = 0 or 1) and cerebrovascular/ cardiovascular risk factors
| Cerebrovascular/ cardiovascular risk factors |
Model 1 | Model 2 | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Diabetes | ||||
| No | Reference | Reference | ||
| Yes | 1.01 | 0.59 – 1.72 | 1.10 | 0.62 – 1.95 |
| Hypertension | ||||
| No | Reference | Reference | ||
| Yes | 0.84 | 0.55 – 1.29 | 0.89 | 0.57 – 1.40 |
| Hypercholesterolemia | ||||
| No | Reference | Reference | ||
| Yes | 0.94 | 0.65 – 1.37 | 1.09 | 0.74 – 1.62 |
| Heart attack/cardiac arrest | ||||
| No | Reference | Reference | ||
| Yes | 1.68 | 0.34 – 9.13 | 2.36 | 0.40 – 15.76 |
| Congestive heart failure present | ||||
| No | Reference | Reference | ||
| Yes | 1.24 | 0.56 – 2.81 | 1.14 | 0.51 – 2.63 |
Model 1 is with the adjustment of age at death and sex, and Model 2 is with the additional adjustment of the number of the ε4 alleles in APOE and four categorized Braak NFT stage.
Table 7.
Associations between ARTAG (NPARTAG = 0 or 1) and cognitive assessment scores.
| Cognitive test | N available out of total N=504 in sample* |
Model 1** | Model 2** | ||||
|---|---|---|---|---|---|---|---|
| ARTAG | Non-ARTAG | P-value** | P-value** | ||||
| MMSE | 79 | 88 | −2.06 (1.07) | 0.055 | −1.76 (0.96) | 0.070 | |
| Logical Memory – immediate | 78 | 110 | −1.42 (0.96) | 0.14 | −0.89 (0.86) | 0.30 | |
| Logical Memory – delayed | 76 | 105 | −1.47 (1.03) | 0.16 | −0.91 (0.93) | 0.33 | |
| Animal Naming | 80 | 114 | −1.84 (1.02) | 0.073 | −1.43 (0.96) | 0.14 | |
| Vegetable Naming | 79 | 110 | −0.52 (0.83) | 0.53 | −0.17 (0.78) | 0.83 | |
| Digit span forward trials | 81 | 112 | 0.25 (0.36) | 0.49 | 0.40 (0.35) | 0.25 | |
| Digit span backward trials | 80 | 112 | 0.39 (0.39) | 0.32 | 0.54 (0.37) | 0.14 | |
N=441 participants had final clinic visit within 6 years between the last follow-up visit and death
Model 1 is with the adjustment of age at death, sex, and years between the last visit and death, and Model 2 is with the additional adjustment of the number of the ε4 alleles in APOE and four categorized Braak NFT stage.
MMSE = Mini Mental State Examination; = Estimate of regression coefficient; SE = Standard error; **−P-value applied two-tailed test
4. Discussion
The main goal of the current study was to assess associations between ARTAG and other brain pathologies in the NACC Neuropathology data set. Data from over 500 autopsied research participants, derived from 20 different ADRCs, were analyzed. In these individuals, the presence of ARTAG was increasingly frequent with more advanced age, with male sex, and was more likely to be found in brains with LATE-NC. ARTAG was also more likely to occur in those with comorbid CVD pathology. However, participants with more severe ADNC, or PART, were not relatively likely to have comorbid ARTAG.
Astrocyte pathobiology and its relationship to dementia is a rapidly expanding field of research (Escartin et al., 2021; Patani et al., 2023; Price et al., 2021), complementing the more widespread emphasis on neuronal degenerative changes. Pathological mechanisms that originate and/or evolve through non-neuronal microenvironments (subpial or white matter regions) may spread, and coexisting pathologies may reflect complex mechanisms: shared upstream risk factors, or direct synergistic influence where one pathology promotes the other. Thus, the present study of comorbid pathologies can provide insights into pathogenesis for both ARTAG and for the other pathologic features seen in aging brains. As suggested by Kovacs et al, “[protein astrogliopathies] might indicate astrocytic contribution to spreading or clearance of disease-associated proteins, however, this might lead to astrocytic dysfunction and eventually contribute to the degeneration of neurons.” (Kovacs et al., 2017a)
Previously published studies found that ARTAG was relatively likely to be observed in brains with comorbid TDP-43 pathology and/or LATE-NC (Prater et al., 2022). For example, Forrest et al (Forrest et al., 2022) showed that ARTAG, and a novel tau pathology termed age-related tau oligodendrogliopathy (ARTOG), were associated with LATE-NC. In a separate Japanese autopsy series of cases lacking severe ADNC, Yokota et al reported that the presence of amygdala granular fuzzy astrocytes was associated with LATE-NC as well as with tau-immunoreactive argyrophilic grains (Yokota et al., 2023). There are other clues that the pathoetiology of astrocytic tau pathology can be linked to TDP-43 proteinopathy. For example, a TARDBP mutation was linked to a pathological phenotype that included ARTAG-like glial tau pathology (Gelpi et al., 2014). Further, a case was identified with GBE1 mutations leading to both ARTAG-like and TDP-43 pathologies (Uemura et al., 2023). Another well-characterized FTLD-linked GRN mutation also showed both ARTAG-like and TDP-43 pathologies (Gomez-Tortosa et al., 2019). In a larger autopsy series, ARTAG was indeed relatively common in brains affected by FTLD-TDP (Koga et al., 2022). Moreover, hippocampal sclerosis of aging has been linked to both LATE-NC and ARTAG (Sordo et al., 2023). The mechanism(s) underlying the synergy or common roots between TDP-43 pathology and ARTAG are currently unknown.
It is notable that ARTAG, has been described to be common in brains affected by main forms of tauopathies, especially progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD) (Kovacs et al., 2017b; Kovacs et al., 2017c; Kovacs et al., 2018a). However, both PSP and CBD are relatively uncommon, with less than 1:100 lifetime risk (Coyle-Gilchrist et al., 2016). The connection between ARTAG and the most prevalent tauopathy – ADNC – is less clear. Kovacs et al reported that ADNC was associated with white matter lobar ARTAG (Kovacs et al., 2017b; Kovacs et al., 2017c). In separate studies by Grinberg and colleagues, ARTAG was found relatively commonly in atypical AD cases (Nolan et al., 2019; Resende et al., 2020), which may represent a set of distinct phenotypes in themselves. The present study did not find any positive association between ARTAG and ADNC severity. With the caveat that the NACC data set has sparse data on lobar ARTAG, this set of observations seems to argue against a general tendency for misfolding proteins to correlate with ARTAG.
The current study’s finding of an association between ARTAG and CVD pathology is novel. It is remarkable that both ARTAG and CVD pathologies become increasingly common with advanced age (unlike ADNC, which levels off and apparently decreases in frequency among persons beyond age 90 years) (Farfel et al., 2019; Nelson et al., 2011; Nelson et al., 2013). However, the positive association that was observed between ARTAG and CVD pathology in the present study was not due to this potential confounder, as age was factored into a regression model as a covariate. We note that in a prior study a positive association was found between Circle of Willis atherosclerosis and ARTAG, but that association did not survive correction in a multivariable statistical model (Kovacs et al., 2018a). The mechanism underlying the association between CVD pathology and ARTAG is presently unknown. Notably, we queried the associations between ARTAG and various cerebrovascular/ cardiovascular risk factors (clinical history of diabetes, hypertension, hypercholesterolemia, myocardial infarction, and heart failure as indicated by self-report at the time of the last clinic visit), and none of these risk factors emerged as having been associated with ARTAG at autopsy. A credible hypothesis is that CVD-linked perturbations (ischemia, hemorrhage, and/or neuroinflammation), due to vascular pathology in aging, promotes astrocyte tau pathology. This may correspond to the known linkages between brain trauma and astrocytic tau pathology (Forrest et al., 2019). Intriguingly, as with the prior study by Kovacs and colleagues (Kovacs et al., 2017b), there was a consistent trend for men’s brains to be affected by ARTAG more frequently than females. This observation could conceivably be linked to sex-linked differential risk for traumatic brain injury (Eom et al., 2021; Gupte et al., 2019). There also may be connections between ARTAG, LATE-NC, and cerebrovascular pathologies (see (Blevins et al., 2020; Harrison et al., 2021; Neltner et al., 2014)). The convergence of medial temporal lobe age-related pathologies suggest overlapping pathogenesis that might include barrier dysfunction (Forrest et al., 2022), an alteration that, together with water-ion imbalances has been suggested to be important factors in the development of ARTAG (Kovacs et al., 2018b). However, these hypotheses need to be tested in future studies.
There were limitations to the current study. There was incomplete data to indicate the location (e.g., subpial, subependymal, white matter, gray matter, anatomical region) and cellular subtypes (e.g. granular-fuzzy or thorn-shaped astrocytes) of the ARTAG. Data on the severity of ARTAG also had a high level of incompleteness. It is hoped that future iterations of the NACC Neuropathology Data Set will have more replete data on ARTAG locations and subtypes. We note that the overall frequency of ARTAG (44% of the study sample) in the present study comports relatively well with prior studies, which suggests that there was not an extremely large number of false-negatives. Beyond the shortcomings to the ARTAG-specific data, there are relatively well-known challenges in population representativeness of autopsy cohorts, and specifically as applies to the NACC Neuropathology Data Set: All 20 ADRCs from which cases were obtained have some overlapping biases including relative paucity of Blacks and Hispanics, as well as exclusion criteria (lack of substance abuses, lack of some neuropsychiatric changes) that make the research participants studied not representative of a broader population(Arce Renteria et al., 2023; Gauthreaux et al., 2023). Furthermore, the academic clinics from which these cases derived were indeed “AD research centers”, and both the clinics and the participants themselves may be selected based on interest in AD/amnestic dementia. Biases of “Alzheimer’s-type” dementia may overlook some other common dementia types such as Lewy body diseases and subtypes of CVD (Gauthreaux et al., 2023). We also note that the data are not very quantitative and some parameters for which numeric data (e.g., number of microinfarcts rather than a dichotomous Yes/No parameter for their presence/absence) would have been more optimal. The sample size was not large enough to discover small sample-size phenomena and this may relate to both pathological and clinical phenomena studied. For example, it is important to note that the cerebrovascular pathologies but not the clinical cardiovascular risk factors were linked to ARTAG pathology, applying the P<0.05 statistical cutoff. However, there was an odds ratio of ~2 for the association between myocardial infarction and ARTAG. In the future, a study with larger sample sizes may enable a more confident test of the null hypothesis.
In conclusion, an analysis of aggregated data sourced from several dozen academic research centers provided fresh insights into associations between ARTAG and co-pathologies in the aging brain. ARTAG was more likely to occur in brains with comorbid brain infarcts, and in males. It remains to be seen whether the association between ARTAG and CVD pathology is related to local infarction-related biochemical signaling, or with some other set of correlated factors.
Supplementary Material
Acknowledgments:
We are extremely grateful to the patients, clinicians, and staff who contributed to this work.
Funding was from NIA/NIH grants P30 AG072946, U24 NS133945, R01 AG061111, RF1 NS118584, R56AG057191, R01AG057187, R21AG061551, R01AG054060, P01AG078116.
The NACC database is funded by NIA/NIH Grant U24 AG072122. NACC data are contributed by the NIA-funded ADCs: P50 AG005131 (PI James Brewer, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG005138 (PI Mary Sano, PhD), P50 AG005142 (PI Helena Chui, MD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005681 (PI John Morris, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG008051 (PI Thomas Wisniewski, MD), P50 AG008702 (PI Scott Small, MD), P30 AG010124 (PI John Trojanowski, MD, PhD), P30 AG010129 (PI Charles DeCarli, MD), P30 AG010133 (PI Andrew Saykin, PsyD), P30 AG010161 (PI David Bennett, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG013846 (PI Neil Kowall, MD), P30 AG013854 (PI Robert Vassar, PhD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P30 AG019610 (PI Eric Reiman, MD), P50 AG023501 (PI Bruce Miller, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P30 (PI Linda Van Eldik, PhD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P30 AG035982 (PI Russell Swerdlow, MD), P50 AG047266 (PI Todd Golde, MD, PhD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG049638 (PI Suzanne Craft, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG066546 (PI Sudha Seshadri, MD), P20 AG068024 (PI Erik Roberson, MD, PhD), P20 AG068053 (PI Marwan Sabbagh, MD), P20 AG068077 (PI Gary Rosenberg, MD), P20 AG068082 (PI Angela Jefferson, PhD), P30 AG072958 (PI Heather Whitson, MD), P30 AG072959 (PI James Leverenz, MD).
Abbreviations:
- AD
Alzheimer’s disease
- ADNC
Alzheimer’s disease neuropathologic change
- ADRCs
Alzheimer’s Disease Research Centers
- APOE
Apolipoprotein E
- ARTAG
age related tau astrogliopathy
- CDR®
Clinical Dementia Rating Dementia Staging Instrument
- CVD
Cerebrovascular disease
- CTE
chronic traumatic encephalopathy
- DLB
Dementia with Lewy bodies
- FTD
Frontotemporal dementia
- FTLD
Frontotemporal lobar degeneration
- FTLD-TDP
Frontotemporal lobar degeneration with TDP-43 inclusions
- LATE-NC
Limbic-predominant age-related TDP-43 encephalopathy neuropathologic change
- MCI
Mild cognitive impairment
- NACC
National Alzheimer’s Coordinating Center
- PART
primary age-related tauopathy
- pTau
Phosphorylated tau protein
- TBI
Traumatic brain injury
- TDP-43
TAR DNA-binding protein 43
- UDS
Uniform Data Set
References cited
- Arce Renteria M., et al. , 2023. Representativeness of samples enrolled in Alzheimer's disease research centers. Alzheimers Dement (Amst). 15, e12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attems J., et al. , 2021. Neuropathological consensus criteria for the evaluation of Lewy pathology in post-mortem brains: a multi-centre study. Acta Neuropathol. 141, 159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachstetter AD, et al. , 2021. Space-occupying brain lesions, trauma-related tau astrogliopathy, and ARTAG: a report of two cases and a literature review. Acta Neuropathol Commun. 9, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekly DL, et al. , 2007. The National Alzheimer's Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 21, 249–58. [DOI] [PubMed] [Google Scholar]
- Besser L., et al. , 2018a. Version 3 of the National Alzheimer's Coordinating Center's Uniform Data Set. Alzheimer Dis Assoc Disord. 32, 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser LM, et al. , 2018b. The Revised National Alzheimer's Coordinating Center's Neuropathology Form-Available Data and New Analyses. J Neuropathol Exp Neurol. 77, 717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieniek KF, et al. , 2021. The Second NINDS/NIBIB Consensus Meeting to Define Neuropathological Criteria for the Diagnosis of Chronic Traumatic Encephalopathy. J Neuropathol Exp Neurol. 80, 210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins BL, et al. , 2020. Brain arteriolosclerosis. Acta Neuropathol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E, 1991. Alzheimer's disease affects limbic nuclei of the thalamus. Acta Neuropathol. 81, 261–8. [DOI] [PubMed] [Google Scholar]
- Butler M., et al. , 2022. Tau Pathology in Chronic Traumatic Encephalopathy is Primarily Neuronal. J Neuropathol Exp Neurol. 81, 773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chornenkyy Y., et al. , 2019. Tau and TDP-43 proteinopathies: kindred pathologic cascades and genetic pleiotropy. Lab Invest. 99, 993–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle-Gilchrist IT, et al. , 2016. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 86, 1736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S., et al. , 1996. Memory improvement following induced hyperinsulinemia in Alzheimer's disease. Neurobiol Aging. 17, 123–30. [DOI] [PubMed] [Google Scholar]
- Crary JF, et al. , 2014. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 128, 755–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di J., et al. , 2023. Urinary Incontinence in a Community-Based Autopsy Cohort Is Associated with Limbic Predominant Age-Related TDP-43 Encephalopathy Neuropathologic Changes. J Alzheimers Dis. 94, 333–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom KS, et al. , 2021. Gender differences in adult traumatic brain injury according to the Glasgow coma scale: A multicenter descriptive study. Chin J Traumatol. 24, 333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin C., et al. , 2021. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci. 24, 312–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farfel JM, et al. , 2019. Alzheimer's disease frequency peaks in the tenth decade and is lower afterwards. Acta Neuropathol Commun. 7, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, et al. , 1975. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 12, 189–98. [DOI] [PubMed] [Google Scholar]
- Forrest SL, et al. , 2019. Chronic Traumatic Encephalopathy (CTE) Is Absent From a European Community-Based Aging Cohort While Cortical Aging-Related Tau Astrogliopathy (ARTAG) Is Highly Prevalent. J Neuropathol Exp Neurol. 78, 398–405. [DOI] [PubMed] [Google Scholar]
- Forrest SL, et al. , 2022. Association of glial tau pathology and LATE-NC in the ageing brain. Neurobiol Aging. 119, 77–88. [DOI] [PubMed] [Google Scholar]
- Gauthreaux K., et al. , 2023. Different cohort, disparate results: Selection bias is a key factor in autopsy cohorts. Alzheimers Dement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelpi E., et al. , 2014. TARDBP mutation p.Ile383Val associated with semantic dementia and complex proteinopathy. Neuropathol Appl Neurobiol. 40, 225–30. [DOI] [PubMed] [Google Scholar]
- Gomez-Tortosa E., et al. , 2019. Presence of tau astrogliopathy in frontotemporal dementia caused by a novel Grn nonsense (Trp2*) mutation. Neurobiol Aging. 76, 214 e11–214 e15. [DOI] [PubMed] [Google Scholar]
- Gupte R., et al. , 2019. Sex Differences in Traumatic Brain Injury: What We Know and What We Should Know. J Neurotrauma. 36, 3063–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison WT, et al. , 2021. Limbic-predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC) is independently associated with dementia and strongly associated with arteriolosclerosis in the oldest-old. Acta Neuropathol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida MA, et al. , 2021. Predictors of cognitive impairment in primary age-related tauopathy: an autopsy study. Acta Neuropathol Commun. 9, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K., et al. , 1998. Glial tau pathology in neurodegenerative diseases: their nature and comparison with neuronal tangles. Neurobiol Aging. 19, S85–91. [DOI] [PubMed] [Google Scholar]
- Ikeda K., et al. , 1995. Thorn-shaped astrocytes: possibly secondarily induced tau-positive glial fibrillary tangles. Acta Neuropathol. 90, 620–5. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, 2022. Recent update on the heterogeneity of the Alzheimer's disease spectrum. J Neural Transm (Vienna). 129, 1–24. [DOI] [PubMed] [Google Scholar]
- Kapasi A., et al. , 2017. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 134, 171–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga S., et al. , 2022. Concurrent tau pathologies in frontotemporal lobar degeneration with TDP-43 pathology. Neuropathol Appl Neurobiol. 48, e12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG, et al. , 2016. Aging-related tau astrogliopathy (ARTAG): harmonized evaluation strategy. Acta Neuropathol. 131, 87–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG, et al. , 2017a. Protein astrogliopathies in human neurodegenerative diseases and aging. Brain Pathol. 27, 675–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG, et al. , 2013. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta Neuropathol. 126, 365–84. [DOI] [PubMed] [Google Scholar]
- Kovacs GG, et al. , 2017b. Evaluating the Patterns of Aging-Related Tau Astrogliopathy Unravels Novel Insights Into Brain Aging and Neurodegenerative Diseases. J Neuropathol Exp Neurol. 76, 270–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG, et al. , 2017c. Multisite Assessment of Aging-Related Tau Astrogliopathy (ARTAG). J Neuropathol Exp Neurol. 76, 605–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG, et al. , 2018a. Sequential stages and distribution patterns of aging-related tau astrogliopathy (ARTAG) in the human brain. Acta Neuropathol Commun. 6, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG, et al. , 2018b. Connexin-43 and aquaporin-4 are markers of ageing-related tau astrogliopathy (ARTAG)-related astroglial response. Neuropathol Appl Neurobiol. 44, 491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAleese KE, et al. , 2016. Post-mortem assessment in vascular dementia: advances and aspirations. BMC Med. 14, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, et al. , 2009. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 68, 709–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirra SS, 1997. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer's disease: a commentary. Neurobiol Aging. 18, S91–4. [DOI] [PubMed] [Google Scholar]
- Mirra SS, et al. , 1991. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 41, 479–86. [DOI] [PubMed] [Google Scholar]
- Mock C., et al. , 2020. The Utility of the National Alzheimer's Coordinating Center's Database for the Rapid Assessment of Evolving Neuropathologic Conditions. Alzheimer Dis Assoc Disord. 34, 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsell SE, et al. , 2016. Results From the NACC Uniform Data Set Neuropsychological Battery Crosswalk Study. Alzheimer Dis Assoc Disord. 30, 134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine TJ, et al. , 2012. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 123, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, et al. , 1989. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 39, 1159–65. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, et al. , 2005. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 53, 695–9. [DOI] [PubMed] [Google Scholar]
- Nelson PT, et al. , 2019. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 142, 1503–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, et al. , 2011. Alzheimer's disease is not "brain aging": neuropathological, genetic, and epidemiological human studies. Acta Neuropathol. 121, 571–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, et al. , 2013. Hippocampal sclerosis of aging, a prevalent and high-morbidity brain disease. Acta Neuropathol. 126, 161–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neltner JH, et al. , 2014. Arteriolosclerosis that affects multiple brain regions is linked to hippocampal sclerosis of ageing. Brain. 137, 255–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan A., et al. , 2019. Astrocytic Tau Deposition Is Frequent in Typical and Atypical Alzheimer Disease Presentations. J Neuropathol Exp Neurol. 78, 1112–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patani R., et al. , 2023. Functional roles of reactive astrocytes in neuroinflammation and neurodegeneration. Nat Rev Neurol. 19, 395–409. [DOI] [PubMed] [Google Scholar]
- Prater KE, et al. , 2022. Glial TDP-43 and TDP-43 induced glial pathology, focus on neurodegenerative proteinopathy syndromes. Glia. 70, 239–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price BR, et al. , 2021. Reactive astrocytes: The nexus of pathological and clinical hallmarks of Alzheimer's disease. Ageing Res Rev. 68, 101335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, R: A language and environment for statistical computing. R. Foundation for Statistical Computing, Vienna, Austria, 2021. [Google Scholar]
- Resende EPF, et al. , 2020. Language and spatial dysfunction in Alzheimer disease with white matter thorn-shaped astrocytes. Neurology. 94, e1353–e1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JT, 2007. Measures of short-term memory: a historical review. Cortex. 43, 635–50. [DOI] [PubMed] [Google Scholar]
- Robinson JL, et al. , 2018. Non-Alzheimer's contributions to dementia and cognitive resilience in The 90+ Study. Acta Neuropathol. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz C., et al. , 2004. High prevalence of thorn-shaped astrocytes in the aged human medial temporal lobe. Neurobiol Aging. 25, 397–405. [DOI] [PubMed] [Google Scholar]
- Skrobot OA, et al. , 2016. Vascular cognitive impairment neuropathology guidelines (VCING): the contribution of cerebrovascular pathology to cognitive impairment. Brain. 139, 2957–2969. [DOI] [PubMed] [Google Scholar]
- Sordo L., et al. , 2023. Characterization of hippocampal sclerosis of aging and its association with other neuropathologic changes and cognitive deficits in the oldest-old. Acta Neuropathol. 146, 415–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DR, et al. , 2002. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 58, 1791–800. [DOI] [PubMed] [Google Scholar]
- Uemura MT, et al. , 2023. Abundant copathologies of polyglucosan bodies, frontotemporal lobar degeneration with TDP-43 inclusions and ageing-related tau astrogliopathy in a family with a GBE1 mutation. Neuropathol Appl Neurobiol. 49, e12865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D., 1987. Wechsler Memory Scale-Revised. Psychological Corporation, San Antonio, TX. [Google Scholar]
- Weintraub S., et al. , 2009. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 23, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagita K., et al. , 2022. A Comparative Study of Site-Specific Distribution of Aging-Related Tau Astrogliopathy and Its Risk Factors Between Alzheimer Disease and Cognitive Healthy Brains: The Hisayama Study. J Neuropathol Exp Neurol. 81, 106–116. [DOI] [PubMed] [Google Scholar]
- Yokota O., et al. , 2023. Amygdala granular fuzzy astrocytes are independently associated with both LATE neuropathologic change and argyrophilic grains: a study of Japanese series with a low to moderate Braak stage. Acta Neuropathol Commun. 11, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.