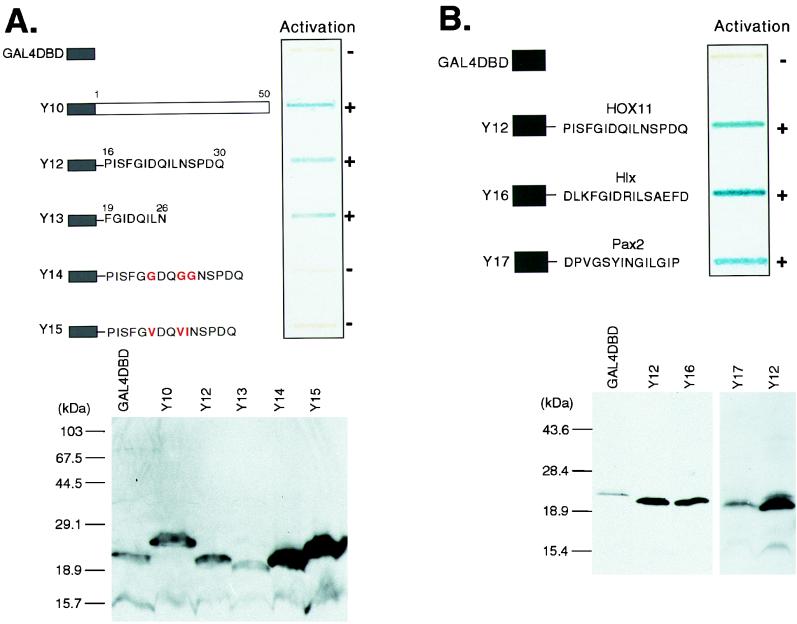
FIG. 3.
The Hep sequence can function as a sequence-specific activation domain. (A) The upper panel shows a yeast β-galactosidase slot blot assay of GAL4-DBD-Hep fusions. Fusions Y10 and Y12 to Y15 are represented schematically. The GAL4-DBD is fused to amino acids 1 to 50 of HOX11 (Y10), amino acids 16 to 30 of HOX11 (Y12), and amino acids 19 to 26 of HOX11 (Y13). Y14 and Y15 are based on Y12 but contain the amino acid substitutions shown. After incubation of the β-galactosidase assay mixture for 2 h, the ability of each fusion to activate the lacZ reporter gene was assessed by the blue color as indicated (when exposed for 12 h, Y14 and Y15 yielded blue coloration comparable to the 2-h exposure of Y12 and Y13). The lower panel shows Western blot detection of GAL4-DBD-HOX11 fusions in yeast extracts with a monoclonal GAL4-DBD antiserum. (B) The ability to activate in yeast is a conserved function of the Hep/octapeptide motif. The upper panel shows the results of a yeast β-galactosidase assay of GAL4-DBD-Hep/octapeptide fusions. The fusions Y12, Y16, and Y17 are represented schematically. GAL4-DBD is fused to a 15-amino-acid peptide encompassing the HOX11 Hep motif (Y12), the Hlx Hep motif (Y16), or the octapeptide sequence from Pax-2 (Y17). The lower panel shows Western blot detection of GAL4-DBD-Hep/octapeptide fusions in yeast extracts with a monoclonal GAL4-DBD antiserum.