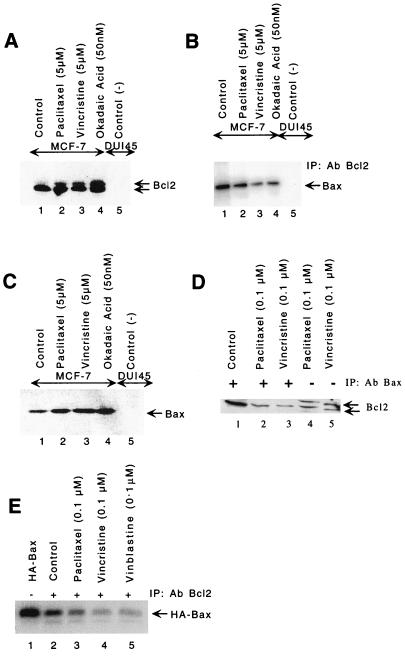
FIG. 3.
Bcl2 hyperphosphorylation and induction of Bax in MCF-7 cells. Paclitaxel (5 μM), vincristine (5 μM), and okadaic acid (50 nM) induce Bcl2 hyperphosphorylation at 6 h (A) and Bax protein levels at 18 h (C). Cells were treated with drugs, and equal amounts of protein were resolved by SDS-PAGE (12% gel) followed by immunoblotting with anti-Bcl2 antibody (A) or anti-Bax antibody (C). (B) Less Bax heterodimerizes with hyperphosphorylated Bcl2 in cell treated with paclitaxel (5 μM), vincristine (5 μM), and okadaic acid (50 nM). Lanes 1 to 4, cell lysates from MCF-7 cells; lane 5, cell lysate from DU145 cells (Bcl2-negative prostate cancer cells). Proteins were immunoprecipitated (IP) with anti-Bcl2 antibody (Ab), resolved by SDS-PAGE (12% gel), and immunoblotted with anti-Bax antibody. (D) Only hypophosphorylated Bcl2 binds to Bax. Cells were treated with paclitaxel or vincristine for 18 h. Lanes 1 to 3, lysates were immunoprecipitated with anti-Bax antibody; lanes 4 and 5, lysates without immunoprecipitation. The blot was probed with anti-Bcl2 antibody. (E) Reduction in heterodimerization between hyperphosphorylated Bcl2 and in vitro-translated HA-tagged Bax. The HA-tagged Bax was cotranslated in vitro in the presence of [35S]methionine and then incubated with cell lysates treated with paclitaxel, vincristine, or vinblastine, immunoprecipitated with anti-Bcl2 antibody, resolved by SDS-PAGE (12% gel), and analyzed by autoradiography. The intensities of hyper- and hypophosphorylated bands were quantitated by densitometry using NIH image software.