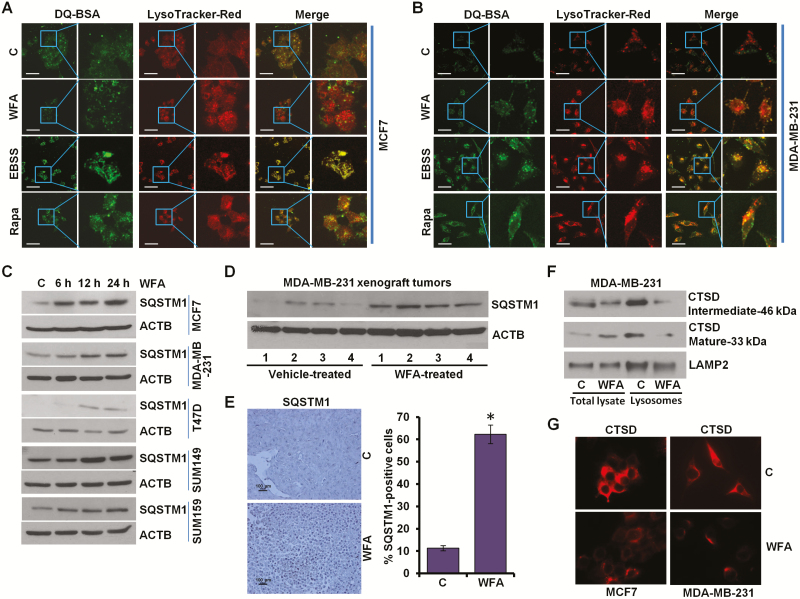
Figure 4.
WFA inhibits protein degradation in lysosomes. (A, B) MCF7 and MDA-MB-231 cells were incubated with 10 μg/ml DQ-BSA for 2 h followed by washing with medium and treatment with 5 µM WFA or 200 nM rapamycin or starving in Earle’s balanced salt solution. The cells were fixed and stained with LysoTracker Red followed by confocal microscopy. Earle’s balanced salt solution and rapamycin were used as positive controls for autophagic induction. Representative fluorescent images are shown. Scale bars: 10 μM. (C) Breast cancer cells were treated with 5 µM WFA for indicated time intervals and total lysates were immunoblotted for SQSTM1 (62 kDa) expression levels. ACTB (45 kDa) was used as loading control. (D) Total protein lysates from MDA-MB-231-derived xenograft tumors from vehicle-treated and WFA-treated mice were examined for the expression of SQSTM1 (62 kDa). ACTB (45 kDa) was used as a loading control. (E) Tumors from vehicle and WFA-treated mice were subjected to immunohistochemical (IHC) analysis using SQSTM1 antibodies. Scale bars: 100 µm. Bar diagrams show quantification of IHC analysis. *P < 0.01, compared with control. (F) MDA-MB-231 and SUM159 breast cancer cells were treated with 5 µM WFA followed by lysosome extraction. Total lysates and lysosomes were immunoblotted for cathepsin D (CTSD). LAMP2 (130 kDa) was used as control. (G) Immunocytochemical analysis of cathepsin D in breast cancer cells treated with WFA.