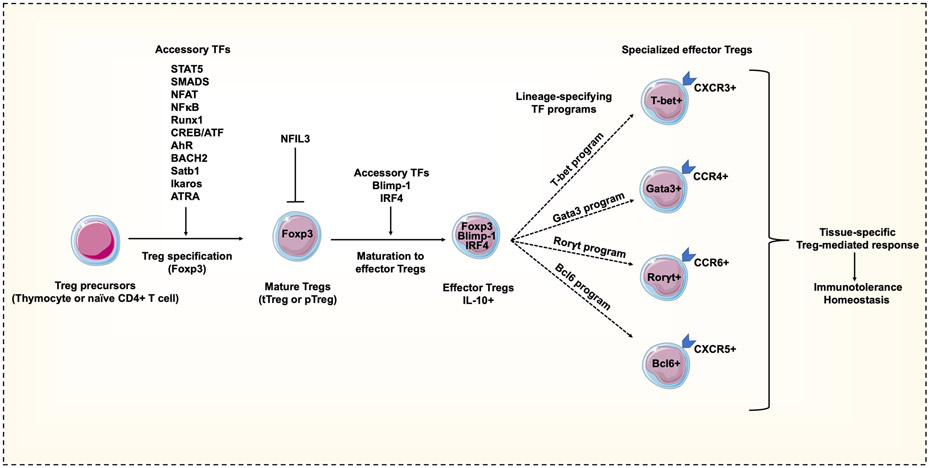
Figure 1. Accessory transcription factors in regulatory T cell specification and maturation.
Thymocytes or naive CD4+ T cells differentiate into regulatory T (Treg) cells following T cell receptor (TCR) engagement within microenvironments rich in Treg cell-inducing soluble factors, such as interleukin-2 (IL-2) and transforming growth factor-β (TGFβ). The coordinated integration of multiple accessory transcription factors (TFs) drive Treg cell specification and epigenetic changes (such as at the Treg cell-specific demethylated region (TSDR)) that are indispensable for stable expression of forkhead box protein 3 (FOXP3). Treg cell maturation to effector Treg cells is driven by FOXP3-dependent and FOXP3-independent accessory TFs that induce FOXP3 expression and enhance production of effector (suppressive) cytokines. Some TFs, such as NFIL3, have the ability to repress FOXP3 expression. Mature effector Treg cells can also be induced to express additional accessory programmes driven by TFs usually associated with lineage-specification in conventional T cells. These shape the unique features of specialized subpopulations of Treg cells, such as tissue homing.