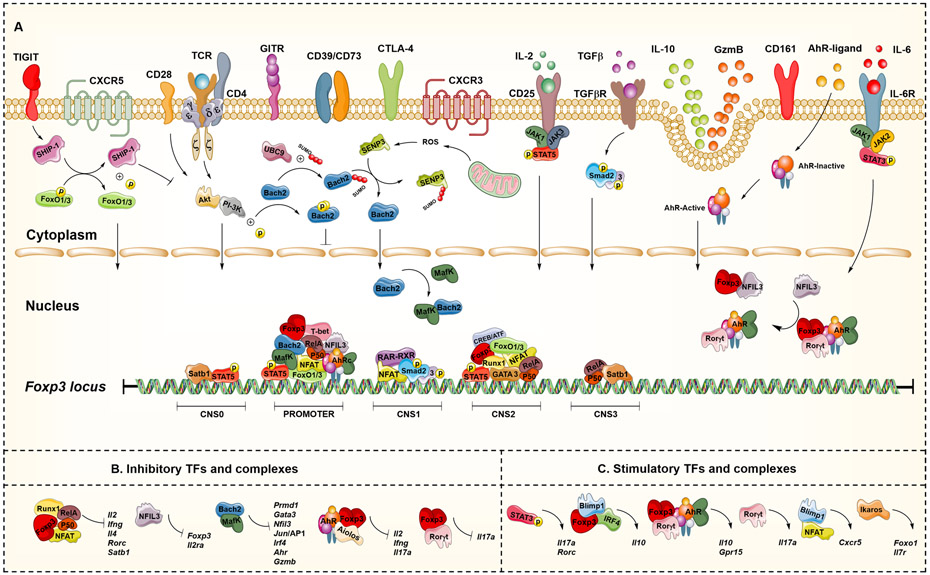
Figure 2. The coordinated network of accessory and lineage-specifying transcription factors regulating FOXP3 expression.
Engagement of T cell receptors (TCRs) by antigen-presenting cells (APCs) through the MHC class II-antigen complex, signalling of interleukin-2 (IL-2) via the CD25-STAT5 module, and activation of canonical transforming growth factor-β (TGFβ)-dependent SMAD pathways all work together to promote the differentiation of regulatory T cells (Treg cells) and expression of forkhead box protein 3 (FOXP3). Other signals, such as cytokines and endogenous chemical compounds present in the environment, are detected by specific cell-surface receptors and transcription factors, such as AHR and RORγt. These signals are integrated along with additional transcriptional regulators (such as BACH2, MAF, Ikaros, Aiolos, RUNX1, FOXO1, SATB1 and NFIL3) at conserved noncoding sequence (CNS) regions of FOXP3. Together, these regulate expression of FOXP3 and the intracellular mechanisms required to activate Treg cell suppressive functions through cell contact or soluble factors. These transcriptional regulators also form stimulatory and inhibitory complexes that regulate genes involved in the maintenance of hallmark and specialized genes expressed by Treg cells, as summarized in Table 2.