Abstract
Dental caries, a highly prevalent oral disease, impacts a significant portion of the global population. Conventional approaches that indiscriminately eradicate microbes disrupt the natural equilibrium of the oral microbiota. In contrast, biointervention strategies aim to restore this balance by introducing beneficial microorganisms or inhibiting cariogenic ones. Over the past three decades, microbial preparations have garnered considerable attention in dental research for the prevention and treatment of dental caries. However, unlike related pathologies in the gastrointestinal, vaginal, and respiratory tracts, dental caries occurs on hard tissues such as tooth enamel and is closely associated with localized acid overproduction facilitated by cariogenic biofilms. Therefore, it is insufficient to rely solely on previous mechanisms to delineate the role of microbial preparations in the oral cavity. A more comprehensive perspective should involve considering the concepts of cariogenic biofilms. This review elucidates the latest research progress, mechanisms of action, challenges, and future research directions regarding probiotics, prebiotics, synbiotics, and postbiotics for the prevention and treatment of dental caries, taking into account the unique pathogenic mechanisms of dental caries. With an enhanced understanding of oral microbiota, personalized microbial therapy will emerge as a critical future research trend.
Subject terms: Applied microbiology, Antimicrobials, Biofilms
Introduction
Dental caries represents a substantial and pressing global public health challenge, affecting a staggering number of individuals worldwide. Specifically, there are an estimated 64.6 million cases of permanent dentition and an additional 62.9 million cases of primary dentition1. Streptococcus mutans, one of the major causative bacteria of dental caries that expresses collagen-binding protein, can effectively invade human umbilical vein endothelial cells2, thereby leading to the potential development of infective endocarditis.
As dental caries is typically mediated by biofilm, interventions targeting biofilm have become a major strategy for prevention. Adjusting the intake of fermentable substrates in the diet, especially sucrose is an effective approach3. The modern dietary environment is characterized by the widespread availability of highly processed and sugary foods, creating a significant challenge in completely abstaining from cariogenic foods. Other interventions include physical clearance (e.g., brushing or using interdental cleaning tools), chemical inhibition (e.g., using chlorhexidine or povidone-iodine), and biological interventions (e.g., using probiotics)4. To effectively prevent dental caries, current strategies should aim to suppress the overgrowth of specific cariogenic bacteria by targeting their virulence factors, while also promoting a diverse and healthy resident microbiota5. Among these interventions, microbial preparations such as probiotics, prebiotics, synbiotics, and postbiotics have gained significant attention as they offer a more targeted and friendly approach than physical clearance and chemical inhibition.
Meurman and colleagues6 were pioneers in introducing probiotics into the field of dentistry. Over time, microbial preparations have gained attention as potential adjunctive therapies for preventing and treating dental caries. These preparations have demonstrated significant effectiveness in inhibiting the growth and biofilm formation of cariogenic bacteria.
This article first provides an overview of the background and pathogenic mechanisms of dental caries, focusing on the virulence factors of cariogenic bacteria S. mutans. It then summarizes the latest research progress, mechanism of action, application status, and challenges associated with the use of probiotics, prebiotics, synbiotics, and postbiotics in the prevention of dental caries. Lastly, this article proposes future directions for the development of this field to provide more scientific, standardized, and effective guidelines for the prevention of dental caries from an academic perspective.
Dental caries
Background
The oral cavity is a complex ecosystem characterized by various warm, moist microenvironments that provide ideal conditions for microbial growth7. A recent study analyzing the oral microbiome identified a total of 1591 microbial species, including bacteria, fungi, archaea, viruses, and protozoa8, second only in complexity to the colon9. The core oral microbiota in healthy individuals remains relatively stable over seven years10, while an imbalanced oral microbiota can lead to dental caries and other oral diseases11. Moreover, the diversity of the oral microbial community in severe dental caries is considerably lower than that in healthy individuals12. Dental caries arises from an imbalance in the oral microbiota resulting from a complex interplay between the host, diet, and microorganisms13.
Of these factors, fermentable carbohydrates, which are commonly found in sweetened foodstuffs, have been identified as particularly important dietary contributors to dental caries14. Consumption of sweetened foodstuffs can rapidly increase the concentration of carbohydrates in the oral cavity, leading to a sharp decline in the pH values of biofilm to 4 or even lower15. Research has revealed a precise correlation between the areas of acute demineralization on the enamel surface and the highly acidic pH zones created by biofilms16. This is attributed to the frequent local pH decreases can disrupt the balance between tooth mineralization and demineralization in the closed microenvironment of the biofilm17. Consequently, this leads to mineral loss in teeth, resulting in white spots, cavitation, pulp infections, and even tooth loss17.
Differences in oral microflora have been observed between individuals with healthy teeth and those with dental caries. For example, the findings of a study on the oral microbiome of children indicate that the genera Rothia, Neisseria, and Haemophilus, which are among the first colonizers of the oral cavity following birth18, are associated with dental health19. In contrast, Prevotella spp., S. mutans, and Human herpesvirus 4 (EB virus) are more commonly found in children with dental caries19. Actinomycetota (35.8%) and Bacillota (31.2%) were the most common phyla in deep dentin carious lesions, and Lactobacillus was the most abundant genus in only 25% of the carious lesions20. There is increasing recognition that dental caries is caused by the imbalanced microbiota in the biofilm, also known as dental plaque, rather than by a single pathogen21.
Microorganisms associated with dental caries
The cariogenic bacteria exhibit varying degrees of contribution to the development of dental caries. For decades, S. mutans and Streptococcus sobrinus have been widely recognized as the major cariogenic agents22. It is noteworthy that S. sobrinus exhibited superior acidogenicity and aciduricity compared to S. mutans, but showed lesser adaptability to the biofilm environment23. The cariogenic bacteria within the oral microbiota do not exist as isolated entities but rather interact and influence each other. To a certain extent, S. mutans creates a lactic acid-rich environment in carious lesions that facilitates the proliferation of Veillonella species12, which have been shown to promote the growth of S. mutans in biofilm studies24.
Additionally, Candida species, as a typical fungal representative of cariogenic microorganisms, have emerged as potent secondary cariogenic agents, isolated from 40% to 60% of adult and pediatric caries25. Candida is a powerful opportunistic caries yeast that relies on the production of short-chain carboxylic acids and proteinases, as well as its ability to adhere to abiotic surfaces and form biofilm25. The most common communication between fungi and bacteria in the oral cavity is the mutual interaction between Candida albicans and S. mutans. The presence of C. albicans promotes the growth of S. mutans, eliciting notable changes in gene expression and enhancing carbohydrate metabolism26. Notably, compared to the mono-species biofilm comprising solely S. mutans, there are 393 differentially expressed genes in S. mutans within the dual-species biofilm26. The glucosyltransferases (Gtf) secreted by S. mutans can bind to C. albicans and facilitate the conversion of sucrose into exopolysaccharide (EPS), thereby providing binding sites for S. mutans27.
One study reported that the core microbiota of early childhood caries (ECC) may include Veillonella parvula, Fusobacterium nucleatum, Prevotella denticola, and Leptotrichia wadei28. On the one hand, this ECC core microbiota promotes the growth and acidogenicity of S. mutans, and promotes biofilm formation, albeit with limited acidogenic capacity28. On the other hand, it also promotes enamel demineralization in vitro and increases the cariogenic potential of enamel in vivo28. Additionally, according to some metagenomic results, the following species are closely associated with dental caries: Streptococcus gordonii, Leptotrichia buccalis, V. parvula, Actinomyces gerencseriae, Propionibacterium acidifaciens, Hallella multisaccharivorax, and Parascardovia denticolens29,30.
Of the microorganisms associated with dental caries, S. mutans is one of the most extensively studied species in this field. Given that S. mutans was initially thought to be a major cause of dental caries31, it is not surprising that most prevention strategies target this bacterium specifically29.
Streptococcus mutans
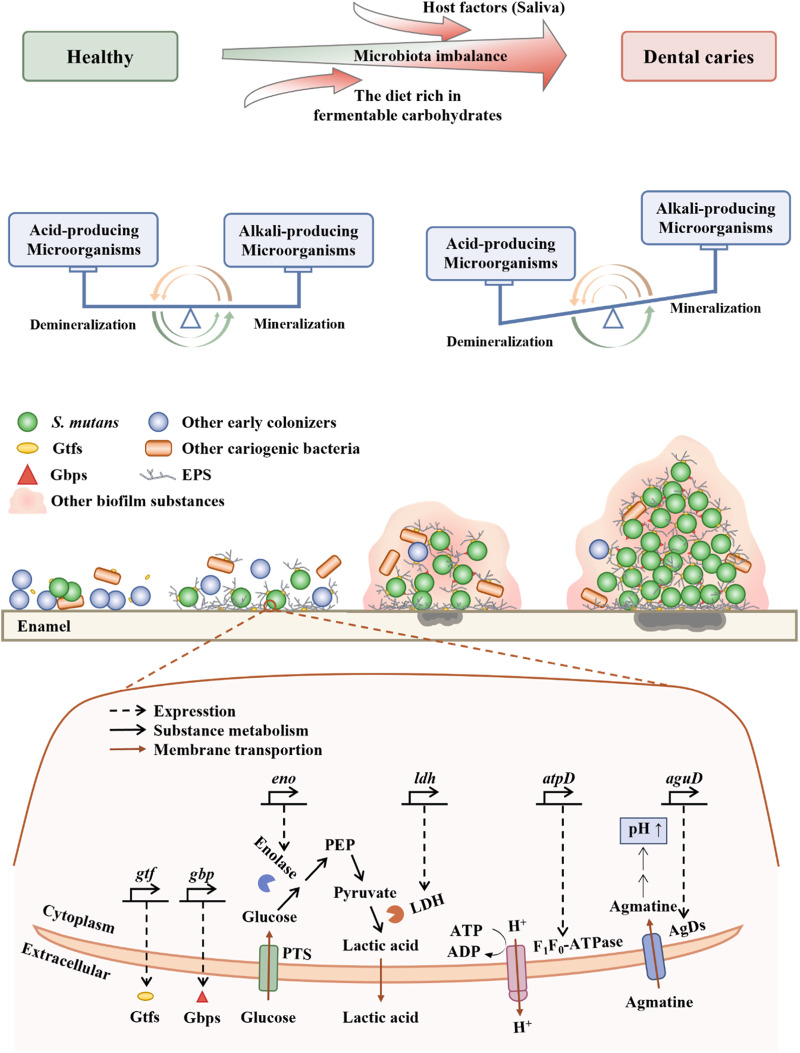
When compared with other original colonizing bacteria, S. mutans exhibits more advantageous traits by developing a compact biofilm and its distinctive virulence factors32. Furthermore, under the regulated control of the quorum-sensing system, S. mutans ultimately becomes one of the major cariogenic bacteria. The biofilm in dental caries of primary teeth is a three-dimensional (3D) spherical structure, with S. mutans as the core and other bacteria forming the outer layer16. This localized area creates an acidic pH environment, leading to severe enamel demineralization16. As dental caries progresses, the diversity of the oral microbiota becomes limited33. This microbial imbalance eventually leads to the occurrence and development of dental caries. The close association between S. mutans and dental caries has been confirmed. Although S. mutans is a natural resident of the human oral cavity34, an increase in the levels of S. mutans should be of concern as it may indicate a clinical precursor to dental caries35. Rats infected with human-derived S. mutans develop dental caries36, and S. mutans may be associated with severe-ECC recurrence37. These suggest the crucial role of S. mutans in the occurrence and development of dental caries. Therefore, further elucidating the pathogenic mechanisms of S. mutans (Fig. 1) is essential for the development of effective strategies for caries prevention and treatment. In the following sections, we will discuss the virulence factors and quorum-sensing system aspect of S. mutans.
Fig. 1. Cariogenic biofilm formation.
EPS exopolysaccharides, Gbps Glucan-binding proteins, gbp encode Gbps, be related to adhesion, Gtfs Glucosyltransferases, gtf encode Gtfs, be related to the synthesis of EPS, eno encode Bacteria enolase, be related to glucose uptake, LDH lactate dehydrogenase, ldh encode LDH, be related to acid production, PTS phosphotransferase, the glucose uptake system, PEP phosphoenolpyruvate, atpD encode F1F0-ATPase, be related to acid resistance, ATP adenosine triphosphate, ADP adenosine diphosphate, AgDs agmatine deiminase system, aguD encode AgDs, be related to acid resistance. Healthy teeth develop dental caries due to the complex interactions between the host, diet, and microorganisms. The microecology of healthy teeth is based on the balance between acidogenic and alkalinogenic microbial activities, as well as the balance between demineralization and remineralization processes. When acidogenic microorganisms become predominant, frequent and high concentrations of acid locally lead to net demineralization of dental enamel, resulting in the formation of cavities. At the micro level, the initial step involves the adhesion of some primitive colonizing microorganisms to the dental enamel. The second step involves the production of EPS by the microorganisms, forming a biofilm. In the third step, acidogenic and acid-tolerant microbial communities, mainly dominated by S. mutans, establish a highly acidic microenvironment, leading to demineralization of the dental enamel. In the fourth step, the highly acidic microenvironment confers a growth advantage to S. mutans-dominated microbial populations. Taking the virulence factors of S. mutans as an example, the synthesis of EPS is primarily mediated by Gtfs. The adhesion process is mainly facilitated by Gbps. Acid production involves the participation of enolase and LDH. Acid tolerance processes primarily rely on the involvement of F1F0-ATPase and AguD.
Virulence factors
The virulence factor of S. mutans can be categorized into four major groups, comprising EPS synthesis, adhesion, acid production, and acid resistance.
The synthesis of EPS
The ability of S. mutans to exert its pathogenicity is largely attributed to the production of EPS. EPS, a major component of biofilms38, consists of extracellular proteins, extracellular DNA, and lipoteichoic acid39. The primary component of EPS is glucan, which is synthesized by Gtf40, providing binding sites for microorganisms38. EPS contributes to the formation of highly organized chemical and physical barriers within the biofilm matrix, facilitating microbial adherence to non-living surfaces41, resisting fluid shear stresses42, evading host immune responses43,44, tolerating antimicrobial agents45, and ultimately establishing and maintaining acidic microenvironment in the oral cavity that favors the development of dental caries-associated biofilm communities42. Mature biofilms are difficult to remove mechanically due to the enhanced viscoelasticity conferred by EPS42. EPS may achieve immune evasion by mediating complement evasion43 and limiting the entry of effector molecules from the innate and adaptive immune systems into the biofilm matrix44. Chlorhexidine, a commonly used antimicrobial agent in oral care, has limited penetration into deep biofilm layers due to its positive charge, whereas “the fuel” (sucrose), lacking charge, can easily diffuse, facilitated by the negative charge of EPS45. The negatively charged surface of S. mutans cells enveloped by EPS accumulates protons, and the sieving effect of the glucan structure also plays a role46. On one hand, EPS captures and accumulates protons produced externally or by acidogenic microorganisms, aiding in the retention and accumulation of acid within the biofilm46. On the other hand, once protons are recruited to the cell surface, they trigger an acid adaptation response, allowing the microorganisms to preemptively counteract acid damage46. Deactivation of one or more gtf genes significantly reduces the virulence of S. mutans, in rodent caries models47. In summary, EPS plays a crucial role in enabling S. mutans to exert its cariogenic potential. Therefore, inhibiting EPS synthesis may represent a feasible preventive strategy against dental caries48.
Adhesion
S. mutans employs both sucrose-independent and sucrose-dependent pathways to adhere to teeth47. The initial adhesion process is primarily mediated by the sucrose-independent pathway, which is subsequently reinforced by the stimulation of glucan synthesis via the sucrose-dependent pathway, ultimately culminating in the formation of biofilms47. Glucan-binding proteins facilitate the binding of glucans synthesized from sucrose through glucose transferases. Of these proteins, GbpA exhibits a strong correlation with cariogenicity49. On the one hand, it contributes to the formation of strong biofilm structure and is an important protein determining the structure of biofilm. On the other hand, it plays an essential role in linking glucan molecules and is involved in the bacterial adhesion process to teeth.
Acid production
After glucose metabolism, dietary carbohydrates produce energy and organic acids as metabolic by-products50. The acid-producing activity of S. mutans is not only a critical factor contributing to its pathogenicity but also a crucial characteristic leading to dental caries. Bacteria enolase, an enzyme encoded by the gene eno, is a primary component of the phosphotransferase system, which is responsible for glucose uptake51. Through the rapid catalytic activity of lactate dehydrogenase (LDH), a protein encoded by the ldh gene, S. mutans UA159 ferments glucose into organic acids52.
Acid resistance
S. mutans employs some acid-resistant mechanisms to cope with the stress of increasing acid production. F1F0-ATPase, a proton pump encoded by atpD47, not only pumps out intracellular protons to maintain intracellular pH but also produces ATP to promote bacterial growth and survival53. Inhibition of atpD expression in S. mutans UA159 resulted in a significant decrease in acid adaptation and an increase in cytoplasmic acidity51. Additionally, S. mutans produces alkali to neutralize acids, as well as export them out of the cells. The agmatine deiminase system plays a crucial role in producing alkalis to overcome acid stress54. Amongst its components, the agmatine-putrescine antiporter (AguD), encoded by the aguD gene, is of particular importance as it facilitates the intracellular transport of free agmatine54. The accumulation of protons on the surface of bacterial cells enveloped by EPS plays a significant role in the acid resistance of S. mutans, as mentioned in the “synthesis of EPS” section46.
Quorum-sensing (QS) system
The QS system regulates virulence and biofilm formation by releasing, sensing, and interacting with diffusion molecules55 based on cell density in the surrounding environment38. S. mutans utilize this system to communicate with each other as a group rather than as separate individuals. The main mechanism for signal feedback is via the two-component signal transduction systems (TCSTS), which enable bacteria to regulate their gene expression56. S. mutans contains several types of TCSTS, among which VicRKX and ComCDE are critical in the regulation of biofilm formation, acid resistance, and acid production in response to environmental signals57,58. If these regulatory systems fail to function properly, it may lead to a decrease in the cariogenicity of S. mutans.
Dental caries prevention measures—biological interventions
Although plaque is a natural occurrence in teeth from an evolutionary, biological, and nutritional perspective, an imbalance in the microbiome of the oral pathological biofilm can lead to the development of dental caries59. Acid-producing cariogenic bacteria, especially S. mutans, damage the hard tooth structures in the presence of fermentable carbohydrates38.
In recent years, the field of biological intervention has developed some novel strategies. One approach involves using predators, such as Bdellovibrio, Bacteriovorax, and Peredibacter, to eliminate anaerobic Gram-negative bacteria that are periodontal pathogens60,61. Given that beneficial bacteria are mostly Gram-positive62. Additionally, biological interventions also include the use of specific inhibitors of S. mutans proteins, vaccination, and passive immunization strategies with neutralizing bacteria25,29. Although some innovative biological intervention strategies such as those mentioned above have emerged, the use of microbial preparations, such as probiotics, prebiotics, synbiotics, and postbiotics, is a more established and popular approach for preventing dental caries.
Probiotics
Background
Probiotics were discovered by scholars as early as 190863, and since then the field of studying the health effects of probiotics on the host has gradually developed. In 2013, The International Scientific Association of Probiotics and Prebiotics (ISAPP) defined probiotics as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host”64. Today, probiotics are commonly used by humans to maintain their overall well-being. Although their effectiveness in promoting gastrointestinal health is well-known, research has also shown that probiotics can be effective in preventing and treating various oral diseases, such as dental caries, oral mucositis, and halitosis65.
In dentistry, probiotics were first introduced by Meurman and colleagues6, who found that Lacticaseibacillus rhamnosus GG ATCC 53103 could colonize the human mouth. With further research, probiotics have been found to have a remarkable ability to prevent dental caries. For instance, one study explored the effect of subjects’ own Lactobacillus on S. mutans66. The study has shown that Lactobacillus isolated from the oral cavity of subjects can effectively inhibit the growth of S. mutans. The most effective species of S. mutans were found to be Lacticaseibacillus paracasei and Lactiplantibacillus plantarum, which are also the most common isolates. Finally, the use of probiotics in the treatment of oral diseases has been found to restore oral microbial balance and reduce the levels of S. mutans in dental plaque and saliva67.
With different probiotic strains exhibiting unique characteristics, understanding the specifics of each strain is crucial to when prevention and treatment of dental caries. For instance, L. rhamnosus GG is a homofermentative Lactobacillus that is not considered to be cariogenic because it cannot ferment sucrose or lactose68. Limosilactobacillus reuteri is an obligate heterofermentative species68 that can produce broad-spectrum antimicrobials with good acid-base stability, such as reuterin69 and reutericyclin70. In addition to Lactobacillus spp., Bifidobacteria spp. may also be a potential probiotic for preventing and treating dental caries. Yogurt containing Bifidobacterium DN-173010 has been reported to significantly reduce the level of S. mutans71.
Mechanisms to prevent dental caries
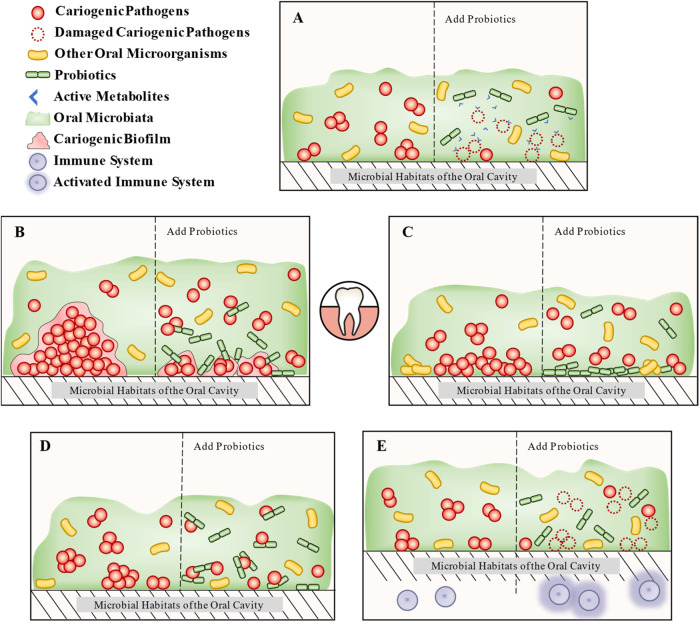
The mechanism through which probiotics can prevent dental caries is similar to that found in the gastrointestinal tract. The principal inhibitory mechanisms include the synthesis of active metabolites, inhibition of cariogenic microbial biofilm, competitive adhesion and colonization, coaggregation with pathogens, and regulation of the immune system (Fig. 2).
Fig. 2. The mechanism of probiotics to prevent dental caries.
It is roughly divided into five parts. A Production of active metabolites: probiotics directly inhibit cariogenic pathogens by active metabolites (e.g., bacteriocin, enzyme, biosurfactants, organic acids, and hydrogen peroxide), which themselves have bacteriostatic activity. B Inhibition of cariogenic microbial biofilm: probiotics can inhibit or remove the biofilm of oral cariogenic microorganisms. C Competitive adhesion and colonization: probiotics not only occupy the colonized sites in the oral cavity but also inhibit the adhesion ability of cariogenic microorganisms. D Coaggregation with pathogens: probiotics inhibit cariogenic microorganisms colonization in the oral cavity through co-aggregation. E Regulation of the immune system: probiotics activate or modulate the host immune system, thereby enhancing the immune response to cariogenic microorganisms (enhances salivary levels of human neutrophil peptides 1–3).
Production of active metabolites
Bacteriocin
Bacteriocin is a cationic antibacterial peptide synthesized by the ribosome72 and was first discovered73. Bacteriocins can be divided into four different classes, with Classes I and II being the primary focus of most probiotics research74. Nisin, a bacteriocin of Type A in Class I, is widely recognized as a small positively charged protein (2–5 kDa) that induces target cells to form membrane pores72. Class II bacteriocins kill bacteria by increasing membrane permeability and leaking target bacterial contents75. In addition to the above mechanisms, bacteriocins can also inhibit the synthesis of biofilm and cell wall, exert the activities of DNase and RNase, and regulate microbiota72.
Protein-protein interaction between the GtfB and LuxS proteins of S. mutans and bacteriocin of SD1 in L. paracasei was found to reduce the formation of biofilm and the density of microorganisms, as revealed in a simulation study76. Following comprehensive bioinformatics analysis and characterization, the bacteriocin in this study was found to be safe for humans. The bacteriocin Mersacidin exerts its bactericidal activity by forming a complex with lipid II, which inhibits cell wall synthesis77. The DNase and RNase activities of colicins from E2 to E9 enable them to non-specifically degrade bacterial DNA and RNA78. Among these, colicin E2 exhibits potent and long-lasting bactericidal activity, and interestingly, it can specifically target bacteria in complex biological membranes79. Bacteriocins are capable of promoting the colonization of producer bacteria in specific niches over a decade, regulating the composition of the microbiota and affecting the host immune system80. Both nisin and nisin-producing probiotics can reduce the level of pathogens in biofilm and restore the diversity of strains to a healthy level81.
As bacteriocins are polypeptides and proteins, temperature control is crucial to ensure their activity during production and use. Streptococcus oralis subsp. dentisani 7746 (AB-Dentisanium®), for instance, is optimally concentrated at 30 and 45 °C, with a small reduction in bacteriocin activity at 60 °C82. This critical consideration underscores the importance of implementing appropriate temperature regulation strategies in the development and use of bacteriocins for various applications.
Enzyme
In addition to bacteriocins, probiotics synthesize a diverse range of enzymes that confer beneficial effects by decomposing biofilms and affecting bacteriocin activity. For instance, Lactobacillus acidophilus can secrete lipase to degrade biofilm83. Similarly, Streptococcus salivarius JH expresses a dextranase enzyme that can hydrolyze the EPS of S. mutans and increase the anti-S. mutans inhibitory activity of zoocin A, a muralytic bacteriocin84. Another example is Streptococcus sp. A12, which produces challisin-like proteases that inhibit the production of bacteriocins by S. mutans85. Additionally, S. salivarius M18 produces urease and dextranase to neutralize salivary acidity and reduce plaque formation, respectively86.
Biosurfactants (BS)
Biosurfactants are amphiphilic substances produced by microbial metabolism that contain both hydrophobic and hydrophilic groups, mainly composed of proteins, sugars, and lipids87. The structure of BS can be identified using various techniques such as thin layer chromatography, Fourier Transform Infrared Spectrometer, and Nuclear Magnetic Resonance88. Fifty percent of the 40 biosurfactant reports reviewed did not analyze their structure, likely due to the complexity of the structures87. Surfactin and/or protein-like biosurfactants (32.5%) are most commonly produced by Lactobacillus, with studies on glycoproteins (7.5%)89, glycolipid (5%)90, and glycolipopeptide (5%)91 production being rare.
Lactobacillus typically produces surfactin-type biosurfactants, which are protein-rich and can significantly inhibit pathogen adhesion, making them increasingly interesting due to their unique anti-adhesion and anti-biofilm properties87. For example, BS produced by L. reuteri DSM 17938, L. acidophilus DDS-1, L. rhamnosus ATCC 53103, and L. paracasei B21060 inhibited S. mutans and Streptococcus oralis adhesion and biofilm formation in a dose-dependent manner on titanium surfaces92. In more detail, L. acidophilus DSM 20079 produces protein-type BS, which can shorten the chain length of S. mutans, interfere with its biofilm formation on glass slides, and down-regulate the gene expression of gtfB and gtfC93. BS produced by L. rhamnosus can destroy the physical structure or protein conformation of biofilm, leading to cell lysis94. In addition to the appealing antimicrobial activity mentioned above, BS exhibits characteristics of low cytotoxicity and high stability. BS derived from Lactobacillus spp. demonstrate comparable low cytotoxicity to rhamnolipids, which are generally regarded as non-toxic products87. BS may exhibit better stability compared to other antibacterial substances produced by probiotics. Gudinahe et al.95 isolated stable BS from L. paracasei. This BS was pH stable within a range of 6–10 and maintained surface activity after incubation at 60 °C for 120 h.
Organic acids
Organic acids, such as lactic acid and butyric acid96, produced by Lactobacillus in the human gastrointestinal tract and other body parts, have widely been recognized as beneficial substances. These organic acids may have a bacteriostatic effect on oral pathogenic microorganisms to a certain degree. For instance, L. paracasei Lpc-37 produces the acid that can restrain the growth and biofilm formation of S. mutans97. However, considering the strong association between dental demineralization and frequent exposure to high concentrations of acid16, it raises questions about how the acids produced by probiotics counteract the acids produced by cariogenic bacteria, including S. mutans.
These questions may need to be approached from the perspective of the overall caries environment. Cariogenic microorganisms create a highly organized acidic barrier42. Prolonged exposure to localized high concentrations of acid leads to localized demineralization rather than average demineralization of the teeth. If the organic acids produced by probiotics can inhibit cariogenic bacteria, including S. mutans, and/or their biofilms, they may disrupt this acidic barrier and prevent acid accumulation. Given the presence of its own acid-base microbial balance in the oral cavity54, acids that do not accumulate but instead contribute to the acid-base equilibrium in the oral environment appear to pose a lesser threat.
Hydrogen peroxide
Certain probiotic species, including Bifidobacterium bifidum, Lactobacillus johnsonii, Lactobacillus crispatus, and Lactobacillus jensenii, produce hydrogen peroxide to exert antibacterial effects98. Hydrogen peroxide acts on the pathogenic bacteria’s epithelium, leading to their death98. Moreover, hydrogen peroxide has the potential to regulate species composition within the oral cavity99. Notably, cariogenic species such as S. mutans are highly susceptible to hydrogen peroxide toxicity100. However, the antibacterial activity of L. paracasei cell-free supernatant (CFS) was significantly reduced after catalase treatment, indicating the involvement of hydrogen peroxide in its bacteriostatic effects101. It should be noted that hydrogen peroxide may not retain its bacteriostatic function after processes such as freeze-drying, owing to the ease of its decomposition102.
Inhibition of cariogenic microbial biofilm
Dental caries is commonly mediated by biofilm. A crucial property of probiotics is the ability to inhibit or eliminate the growth of biofilms and pathogenic microorganisms in the oral cavity. Some strains have often been reported for their anti-biofilm activity, including S. oralis 89a, Limosilactobacillus fermentum TCUESC01, L. acidophilus 4A, and Bifidobacterium longum subsp. longum103. For instance, Lacticaseibacillus casei ATCC 393, L. reuteri ATCC 23272, L. plantarum ATCC 14917, and Ligilactobacillus salivarius ATCC 11741 may suppress the biofilms of S. mutans by down-regulating genes such as gtfB, gtfC, and gtfD in S. mutans104. Interestingly, probiotics are capable of inhibiting fungi from transitioning into pathogenic forms. It has been demonstrated that L. rhamnosus LR32, L. casei L324m, and L. acidophilus NCFM exhibit the ability to impede the initial stages of hyphal formation, which is a crucial step in the pathogenesis of C. albicans105. A probiotic combination consisting of Lactobacillus helveticus CBS N116411, L. plantarum SD5870, and S. salivarius DSM 14685 significantly down-regulated the expression of genes involved in yeast-hypha transition in C. albicans, including EFG1 (hyphae-specific gene activator), SAP5 (secreted protease), ALS3 (adhesin/invasin) and HWP1 (hyphal wall protein)106. In vitro, biofilm models are continuously improving to replicate more closely the conditions found within the human body. Based on the specific research objectives, researchers can opt for models of interest, such as an experimental abutment mimicking the macro- and microstructure of a dental implant107.
The combination of L. rhamnosus and collagen peptides was found to significantly increase the pH of the medium in the early stages of biofilm formation108. The qPCR results showed that this combination down-regulated several crucial genes linked to acid production and acid tolerance, including eno, ldh, and atpD. Moreover, probiotics may also exert antibacterial effects by interfering with QS. A study revealed that comD, vicR, and vicK genes were down-regulated in planktonic and biofilm forms of S. mutans when exposed to CFS with Lactobacillus104. This effect may explain the reduced adherence and biofilm formation of S. mutans observed in scanning electron microscopy experiments.
Competitive adhesion and colonization
One of the key characteristics of probiotics contributing to their health effects is their capacity for outcompeting oral pathogens concerning adhesion and colonization109. For example, L. reuteri LR6 displayed the most substantial adhesion capabilities among eight tested probiotic strains, which corresponded to a higher ability for inhibiting the adherence of pathogens to Caco-2 cells110. Enhanced colonization efficacy by S. salivarius M18 resulted in stronger anti-caries activity as evidenced by a reduction in plaque scores and S. mutans levels111. Levilactobacillus brevis KCCM 202399 inhibited the adherence of S. mutans KCTC 5458 by reducing the self-aggregation, cell surface hydrophobicity, and EPS production of S. mutans112.
Interestingly, probiotics can reduce pathogen adhesion even without direct contact. Saliva treated with probiotics was shown to reduce the adhesion of S. mutans to hydroxyapatite surfaces (a model for enamel)113. Further studies showed that the above salivary membrane lacked two proteins: salivary lectin gp340, the primary receptor for S. mutans in the salivary membrane, and salivary peroxidase, an innate defense factor found in human saliva114.
Coaggregation with pathogens
Coaggregation is among the advantageous properties of probiotics as it allows them to form a barrier that impedes pathogen colonization115. In a study, six out of 624 lactic acid bacteria were found to exhibit specific coaggregation with S. mutans in vitro116. These species were identified as L. paracasei and L. rhamnosus. It was discovered that this coaggregation mechanism is highly resilient to both hyperthermia and protease, and does not rely on lectins, nor is it impacted by saliva.
Regulation of the immune system
In addition to their direct effects on pathogenic microorganisms or biofilm, probiotics are known to activate and modulate the host’s immune system117. Clinical studies have shown that daily or tri-weekly consumption of L. paracasei SD1 in patients with severe ECC significantly enhances salivary levels of human neutrophil peptides 1–3 with a broad bactericidal activity and reduces S. mutans levels, potentially slowing the progression of caries118. Furthermore, the consumption of milk containing L. paracasei SD1 for six months increased salivary immunoglobulin A levels, and this increase is positively correlated with a load of L. paracasei119.
Certain strains of Streptococcus thermophilus, such as ST1342, ST1275, and ST285 activate the innate immune response and stimulate the secretion of interleukin-1β, tumor necrosis factor-α, interleukin-6, and interferon-γ by monocytes, thereby contributing to the elimination of pathogens103. Commercial L. paracasei DG has immunostimulatory activity by boosting tumor necrosis factor-α, interleukin-6, and Chemokine (C-C motif) ligand 20 expressions in human monocyte leukemia cell120. These findings suggest that probiotics can enhance the host’s immune response against pathogenic microorganisms, providing a potential approach to preventing and treating infectious diseases.
The application vehicle
The colonization of probiotics in the oral cavity may be influenced by the choice of the delivery vehicle68. A range of vehicles is available for delivering probiotics, including dairy products, ice cream, cereal, pacifiers, chewing gum, curd, juice, and mouth wash (Table 1). Table 1 also mentions the test species, dose, and efficacies.
Table 1.
Application vehicle and therapeutic effects of probiotics
| Vehicle | Test strain | Dose | Frequency | Sample | Result | Reference |
|---|---|---|---|---|---|---|
| Milk powder | L. paracasssei | 5 × 107 CFU | once daily for 3 months | 124 children aged 1.5–5 | reduced the count of S. mutans in saliva and delayed the development of new dental caries | 187 |
| Milk | L. paracasei | 7.5 × 109 CFU | once daily for 4 weeks | 30 orthodontically treated nonsyndromic cleft lip and palate patients with a mean age of 19 | reduced the count of S. mutans, while increasing the count of Lactobacillus and the colonization | 188 |
| Yogurt | B. animalis | 2 × 108 CFU | once daily for 2 weeks | 49 healthy children aged 6–12 | could not reduce the levels of salivary S. mutans and Lactobacillus | 189 |
| Yogurt | B. lactis | unclear | once daily for 2 weeks | 30 individuals aged 10–30 undergoing orthodontic treatment | reduced total microbial counts in dental plaque | 190 |
| Yogurt | B. lactis BB12 | 1 × 106 CFU | 300 g daily for 2 weeks | 66 students aged 18–30 with initial stages of dental caries | reduced the count of S. mutans and Lactobacillus in the probiotic group | 191 |
| Cheese | L. acidophilus NCFM or L. rhamnosus Lr-32 (DuPontTM Danisco®, São Paulo, Brazil) | 1 × 108-9 CFU/g each strain | 50 g daily for 16 weeks | 60 elderly denture wearers | reduced the colonization of oral Candida | 192 |
| Cheese | L. rhamnosus GG and L. rhamnosus LC705 | 1 × 107 CFU/g each strain | 5 × 15 g daily for 3 weeks after a meal or snack | 74 adults aged 18–35 | reduced the count of S. mutans during the post-treatment period | 193 |
| Cheese | L. casei LAFTIL26 | 1 × 106 CFU/g | 50 g twice daily for 2 weeks with breakfast and dinner meals | 60 adults with a mean age of 28 | could not reduce the count of S. mutans and Lactobacillus in the probiotic group | 194 |
| Ice cream | B. lactis Bb-12 and L. acidophilus La-5 | 1 × 106 CFU each strain | once daily for 7 days | 60 healthy children aged 6–12 | reduced the count of salivary S. mutans | 195 |
| Cereal | L. paracasei F19 | 1 × 108 CFU | once daily for 9 months | 179 infants aged 4 months | no impact on the frequency of dental caries, mutans streptococci, or lactobacilli | 196 |
| Novel slow-release pacifier | B. animalis lactis BB-12 | 1 × 1010 CFU | twice daily for 2 years | 106 infants aged 1–2 months | no impact on the oral colonization of B. animalis lactis BB-12 and mutans streptococci in the early administration | 197 |
| Chewing gum | L. reuteri ATCC 55730 and ATCC PTA 1 | 1 × 108 CFU/gum each strain | three times daily after meals for 3 weeks | 80 healthy adults aged 21–24 | significantly reduced the levels of salivary mutans streptococci | 198 |
| Curd | L. acidophilus and B. lactis BB12 (Mother dairy b-activ Plus®) | unclear | once daily for 7 days before breakfast | 60 caries-free adults aged 20–25 | Significantly improved salivary pH and reduced the count of salivary S. mutans | 199 |
| Curd | L. acidophilus-SD 5221 (Active Plus; Nestle, Chennai, India) | 1 × 109 CFU | with their lunch for 30 days | 60 orthodontic patients aged 14–29 | significantly reduced the levels of S. mutans in the plaque around the brackets | 200 |
| Carrot-pineapple juice (Gefilus®) | L. rhamnosus GG | 5 × 106 CFU/mL | five times a week for 7 months | 530 healthy children aged 3–6 | reduced the count of S. mutans and the risk of dental caries | 201 |
| Mouthwash (ProBiora3,TM]) | S. oralis KJ3sm, S. uberis KJ2sm, and S. rattus JH145 | 106 or 108 CFU each strain | twice daily for 4 weeks | 20 healthy adults aged 21–35 | reduced the levels of S. mutans | 202 |
CFU colony forming units, B. animalis Bifidobacterium animalis, S. uberis Streptococcus uberis, S. rattus Streptococcus rattus.
Among these vehicles, dairy products are considered ideal carriers due to their inherent beneficial characteristics121. Among the dairy products, liquid substrates, such as milk and yogurt, were found to be more effective in reducing S. mutans levels122. For individuals who are allergic to dairy products, alternative carriers may be selected, as illustrated in Table 1. The buffering capacity of milk helps to reduce acid production, while its colloidal nature appears to protect enamel123. In addition, milk contains calcium and calcium lactate, which may have a preventive effect against caries124, and can reduce the colonization of pathogenic microorganisms125. Furthermore, milk and cheese promote the dominance of casein phosphopeptides which are known to play a key role in biomineralization126. A systematic review and meta-analysis showed that dairy products containing probiotics had a significant impact in reducing S. mutans and raising salivary pH122.
Interestingly, the slow release of probiotics can also be achieved using appropriate embedding materials. For instance, L. paracasei 28.4-gellan formulations were recently found to release the probiotic for over 24 h127. L. paracasei in this state was able to inhibit S. mutans in both the floating and biofilm states, significantly reduce the generation of EPS, and downregulate the luxS, brpA, gbpB, and gtfB genes.
Controversy
Some researchers have taken a critical view of the idea that probiotics can prevent dental caries, primarily focusing on its safety and potential cariogenic effects. The majority of probiotics are not derived from the oral microbiota but from fecal samples, and some even come from animals82. Therefore, it is necessary to thoroughly evaluate their safety before clinical use. Probiotics may pose a risk to individuals with damaged barriers or low immunity, such as bacteremia128 because high concentrations of administration are key to medication. Regarding the cariogenic issue, after conducting a meta-analysis of 50 experiments related to dental caries and periodontal diseases, Gruner and colleagues concluded that there is insufficient evidence to support the use of probiotics in treating dental caries129. Subjects with active dental caries showed higher levels of S. mutans, Actinomyces sp. strain B19SC, and Lactobacillus spp. as detected by PCR-based methods130. The major lactic acid bacteria identified from carious lesions, including both adults and children, include L. fermentum, L. casei/paracasei, L. salivarius, L. rhamnosus, L. plantarum, and L. gasseri131. Bifidobacterium dentium, considered a late marker for dental caries progression, was not found in the oral cavity of caries-free individuals but was detected in 30.8% of caries cases in a study of 56 participants132.
It is unreasonable to conclude a causal relationship between lactic acid bacteria and caries if there is a strong correlation between lactic acid bacteria and caries scores133. Lactic acid bacteria have a relatively low affinity for teeth, and their ability to form biofilms in vitro is much weaker than that of S. mutans131. The attachment and proliferation of secondary invaders including Bifidobacterium and lactic acid bacteria requires initiation of caries by major caries promoters including S. mutans to create an anaerobic acidic environment rich in carbohydrates132,133. The destruction of dentin is not enough by lactic acid alone but also requires proteolytic activity, because the main component of dentin134 is more than the extracellular matrix dominated by type I collagen. However, lactic acid bacteria, including L. rhamnosus, L. casei/paracasei, L. salivarius, Lactobacillus vaginalis, Lactobacillus gasseri, Limosilactobacillus oris, and L. fermentum, have a greater propensity to bind to collagen rather than degrade it based on genomic analysis133.
In conclusion, the effectiveness of probiotics in preventing dental caries remains controversial. It is beneficial to prevent dental caries by exploring the roles of each microflora in the transition from oral health microbiota to cariogenic microbiota. In particular, from the perspective of oral microecology, the diet composition, host immune environment, and the physical and chemical characteristics of the oral cavity, especially the teeth, should be fully considered. Therefore, in recent years, researchers have become increasingly interested in exploring the benefits of prebiotics, synbiotics, and postbiotics in preventing dental caries, especially in terms of advantages over probiotics.
Prebiotics
In 1995, prebiotics were defined as “non-digestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria already resident in the colon”135. However, with advancements in scientific research, ISAPP deemed the definition of prebiotics as “a substrate that is selectively utilized by host microorganisms conferring a health benefit” more appropriate in 2017136. Prebiotics present a safe and effective alternative to probiotic intervention since they are not live bacteria and are less susceptible to environmental factors affecting probiotic survival and efficacy. The following section will discuss prebiotics according to their different types, including their mechanisms of action, efficacies, and other related aspects.
Sugar
Interestingly, certain sugars exhibit prebiotic properties. D-tagatose, a non-cariogenic sugar, is a potential prebiotic that offers lower calories and a lower glycemic index than sucrose137. Notably, the saliva of individuals with good oral health is rich in D-tagatose138. D-tagatose may inhibit the growth of S. mutans and S. gordonii by affecting glycolysis and its downstream metabolism, but it does not affect S. oralis138. Encouragingly, chewing gum containing D-tagatose has been shown to inhibit the growth of S. mutans139.
In addition to D-tagatose, other sugars such as xylose and arabinose are considered potential prebiotics, with the capacity to not only inhibit the growth of S. mutans but also promote the growth of Lactobacillus140. This dual action is particularly advantageous, as it may help restore the balance of the oral microbiome. Given the promising prebiotic properties of these sugars, further research is needed to assess their effectiveness in vivo and their potential side effects.
Sugar alcohol
Sugar alcohols, such as xylitol, sorbitol, maltitol, and erythritol, have been shown to exhibit prebiotic properties that can enhance oral health. Xylitol, a five-carbon polyol sweetener141, is considered an oral-specific prebiotic according to the new definition established in 2017142,143. It offers numerous benefits, including the enhancement of remineralization, decrease of the pH of dental plaque, reduction of the level of S. mutans in saliva, reduction of the insoluble dextran in the biofilm of S. mutans, and reduction of dental caries incidence144–146. However, xylitol loses its effect in the presence of fructose or sucrose147. Other sugar alcohols, such as sorbitol, maltitol, and erythritol, have also been shown to inhibit dental caries148,149.
Oligosaccharides
In addition to sugar alcohols, oligosaccharides are also being investigated as prebiotics. Human milk oligosaccharides (HMOs), the third most abundant ingredient in human milk, are often added to infant formula150. Galacto-oligosaccharides (GOS) and 2′-fucosyllactose, the most abundant HMOs, were found to reduce the EPS-mediated adhesion of S. mutans DSM 20523 to the glass surface, indicating their potential as prebiotics for oral health promotion150. In addition, GOS, glucomannan hydrolysates, and mannose have also been shown to inhibit pathogen adhesion to epithelial cells by binding to the pathogen’s lectins/pili151. However, it should be noted that non-digestible and/or non-absorbable sugar alcohols and oligosaccharides, though beneficial for health, excessive intake may lead to significant diarrhea152. Therefore, further research is needed to determine the optimal dosage and duration of intake to minimize such adverse effects.
Arginine
Arginine is a widely studied oral prebiotic that has been shown to exhibit various beneficial effects on oral health38. These benefits include the promotion of alkaline substance production, the mitigation of tooth demineralization, and the suppression of biofilm formation. Specifically, arginine can inhibit the growth of Candida153 and reduce enamel demineralization154. Furthermore, L-arginine has been found to enhance the alkali-producing ability of arginine-solubilizing bacteria, such as Streptococcus sanguinis and S. gordonii155, thereby making the biofilm environment unsuitable for cariogenic microflora by increasing the pH. Interestingly, L-arginine was found to significantly reduce the amount of insoluble EPS by 3-fold, targeting gtfB156.
Numerous studies have used toothpaste containing arginine to explore the mechanism underlying its prebiotic effects on oral health in depth. An in vivo study conducted on the oral ecosystem has revealed that the presence of arginine in toothpaste enhances the arginolytic capacity of human saliva while reducing its sucrose metabolic activity157. Additionally, it promotes a shift in the composition of salivary microbiota towards a healthier ecological state. Notably, tubes of toothpaste containing arginine and fluoride were more effective at preventing and reversing early caries lesions158 and significantly increased re-mineralization compared with fluoride-only toothpaste159. Using toothpaste containing fluoride and arginine was also associated with an increase in gene expression associated with the arginine deiminase pathway, according to metagenomic and metagenomic data160. Moreover, the use of toothpaste containing fluoride and arginine was found to reduce caries bacteria and promote healthier microbial communities.
Interestingly, arginine may stimulate the production of effective substances by probiotics. Exogenous arginine has been found to increase the expression of the S. gordonii spxB gene, which encodes a pyruvate oxidase (SpxB), thereby promoting hydrogen peroxide production156. Additionally, using Mg2+ as a cofactor of the catalytic activity of SpxB161 has been shown to increase the production of H2O2 and promote the abundance of SpxB in S. sanguinis and S. gordonii161.
Urea and nitrates
Urea and nitrates have been investigated as potential oral prebiotics. Urea is considered a prebiotic162 due to its ability to be converted into ammonia or ammonium and bicarbonate ion by bacteria possessing urease, such as S. salivarius, Actinomyces naeslundii, and Haemophilus spp., thereby neutralizing acids in the oral cavity163. Nitrate-reducing bacteria in the oral cavity convert salivary nitrate to nitrite, which is subsequently reduced to nitric oxide164. All three compounds have been shown to restrict the growth of pathogenic bacteria165,166. Nitrate has demonstrated the ability to reduce caries incidence164,165 and inhibit bacteria commonly associated with dental caries, such as S. mutans NCTC 10499, L. casei, and A. naeslundii, as well as periodontal disease-related bacteria including F. nucleatum, Eikenella corrodens, and Porphyromonas gingivalis167. However, it can lead to an increase in the levels of Neisseria and Rothia, genera associated with oral health and nitrate reduction168. Salivary nitrate supports nitrate respiration by anaerobic microorganisms, ultimately increasing oral pH through various mechanisms. These mechanisms include competition for carbon sources with acid-producing fermentation processes, generation of hydroxyl ions, and dissimilation of nitrate into ammonium to consume organic acids168,169. In addition to its impact on dental caries, dietary nitrate not only benefits oral health by significantly reducing gum inflammation170 but also contributes to overall health by lowering systemic blood pressure171,172, promoting vascular health172, and even potentially improving vascular function in patients with hypercholesterolemia173. Considering the systemic health effects of nitrate, further investigation is warranted to explore other mechanisms through which nitrate can prevent dental caries.
Both probiotics and prebiotics have beneficial effects on health and combining them appropriately for co-administration represents another approach to drug administration. The following section will further elaborate on this combination.
Synbiotics
The combined use of probiotics and prebiotics has demonstrated superior therapeutic effects compared to their utilization174. The term “synbiotics” was first coined in 1995 by Gibson et al. to describe the combination of probiotics and prebiotics135. ISAPP updated the definition of synbiotics in 2020, stating that it is a mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host175. It is not surprising that synbiotics have received less attention compared to probiotics, as it was proposed relatively later.
Several studies have indicated potential oral health benefits associated with synbiotic use. For instance, Nunpan et al.176 demonstrated that the synbiotic composed of L. acidophilus in combination with GOS and fructo-oligosaccharides can significantly inhibit the growth of S. mutans. Tester and Al-Ghazzewi177 found that synbiotics composed of Konjac glucomannan hydrolysates and L. acidophilus reduced the levels of S. mutans in vitro. In another study, Kojima et al. proposed a novel symbiotic140. They screened five strains of lactobacilli using sugar assimilation tests with 12 different saccharides, among which the three most promising prebiotics were found to be arabinose, xylose, and xylitol. The selected lactobacilli significantly inhibited the production of water-insoluble glucan by S. mutans140.
Considering the significant role of excessive acid in dental caries development, the selection of synbiotics comprising prebiotics capable of maintaining a high pH oral environment represents an innovative and intelligent approach. Synbiotics composed of 2% L-arginine and L. rhamnosus not only reduced the biomass of S. mutans biofilm but also decreased lactate content in spent media, resulting in no significant decline in pH within 24 h178. This suggests that synbiotics modulate the ecology of dental plaque. Remarkably, this study also observed that the addition of L-arginine promoted the positive utilization of amino acid biosynthetic pathways by L. rhamnosus, thus facilitating its proliferation. These findings indicate that the selection of synbiotics capable of pH regulation may offer stronger advantages in the oral cavity.
These findings provide promising evidence for the development of synbiotics as a novel approach to improving oral health. Further research is needed to establish the safety and efficacy of different synbiotic combinations. It is hoped that these advances will lead to the development of innovative yet effective strategies for promoting oral health, thereby improving overall health outcomes.
Postbiotics
In 2021, ISAPP defined “postbiotics” as “the preparation of inanimate microorganisms and/or their components that confer a health benefit on the host”179, excluding essentially purified metabolites such as butyric acid179. Before this, the term and definition of postbiotics were not officially standardized and unified. Postbiotics have also been referred to as “paraprobiotics”, “heat-killed probiotics”, “ghost probiotics”, “non-viable probiotics” and “bacterial lysates”180. Over the past few years, the research direction of postbiotics has garnered increasing attention from researchers and has gradually become a hot research topic. Postbiotics are considered superior to probiotics due to their good acid–base and thermal stability, ease of storage and use, and high safety, as many probiotics are sensitive to oxygen and heat179,181. The feature of postbiotics enables them to be added to common products such as toothpaste, chewing gum, natto, potato chips, popcorn, and suckable candies179,182. Another advantage of postbiotics is that microorganisms cannot be isolated from commercial products, thus enabling product developers to maintain ownership of their components179.
The main methods of preparing postbiotics are heat inactivation of bacterial cells and preparation of CFS. In addition, other inactivated technologies, such as electric field, ultrasonication, high pressure, X-rays, high voltage electrical discharge, magnetic field heating, moderate magnetic field, and plasma technology, are also available179,183. It should be noted that the mode of inactivation may affect the activity of postbiotics to some extent. For example, in one study, the activity of CFS of S. oralis subsp. dentisani 7746 concentrated at 60 °C was lost, while CFS concentrated at 30 °C or 45 °C retained its activity82. The ability of heat-killed L. reuteri to adhere to and inhibit pathogens was significantly reduced compared to L. reuteri110, possibly due to the alteration of physical and chemical properties caused by heat treatment184.
Although postbiotics do not contain live microorganisms, this does not imply that inactivated bacteria have completely lost all beneficial properties. For example, heat-killed Bifidobacterium animalis BB12 still can reduce the cariogenicity of biofilm in vitro185, indicating that heat-killed bacteria did not completely lose all their beneficial properties. As another example, L. paracasei DSMZ16671 maintained its ability to co-aggregate with S. mutans after heat-killed treatment (autoclaved at 121 °C for 20 min)116. There was even a study that showed that L. rhamnosus CNCM-I-3698 and Companilactobacillus farciminis CNCM-I-3699 had a greater ability to exclude pathogens and adhesion after heat-killed treatment186. The inactivation process may lead to the disruption of bacterial cell structures, making bioactive molecules more exposed and accessible for utilization179. Postbiotics, which are mixtures of various components, may exert their health effects on the oral cavity through multiple mechanisms. These mechanisms can be independent or cooperative and may resemble the previously described mechanisms of probiotic health effects. In the interest of brevity, we will not reiterate these mechanisms here.
In the context of dental caries prevention and treatment, the types of postbiotics commonly utilized include CFS and heat-killed probiotics. However, it is worth mentioning that research in fields beyond the oral cavity has explored a broader range of postbiotic types, such as peptidoglycans, lipopolysaccharides, and pili179. Additional investigations into the mechanisms by which other types of postbiotics act concerning dental caries have the potential to provide innovative approaches for the prevention and treatment of this condition. Furthermore, further studies are warranted to assess the safety and effectiveness of postbiotics, thereby contributing to the improvement of overall health outcomes.
Conclusion and future perspectives
Dental caries prevention and treatment is a critical issue in oral healthcare. The traditional prevention and treatment methods have mainly focused on physical removal and chemical inhibition. While minimally invasive dental restoration can treat cavities, it fails to address the underlying causes of new caries formation. Thus, safer, more effective, and personalized preventive and treatment strategies are urgently needed. Extensive research has been conducted on the application of microbial preparations, such as probiotics, prebiotics, synbiotics, and postbiotics, in the prevention and treatment of dental caries, with promising outcomes. These microbial preparations can modulate the balance of oral microbiota by introducing beneficial microorganisms or inhibiting pathogenic ones. This review aims to help overcome theoretical obstacles to the successful clinical application of microbial preparations in preventing and treating dental caries.
In conclusion, investigating the potential applications of microbial preparations in the prevention and treatment of dental caries is an essential research avenue in the field of oral microbiology. The successful application of microbial preparations in the clinical setting can provide crucial support and greater assurance for oral health. Dental caries pathogenesis is closely associated with the balance of oral microbiota. Therefore, future research should focus on obtaining a deeper understanding of the characteristics and interrelationships among various beneficial and harmful microbial populations. With such comprehensive research, it is advantageous to develop more effective microbial preparations and optimal allocation methods. Developing personalized treatment plans is the current trend, and by devising microbial preparations that are effective for different dental caries risk and medication risk populations, personalized prevention and treatment of dental caries can be achieved. Furthermore, personalized treatment schemes are not limited to single microbial preparations and can be used alongside other treatment modalities to enhance the overall therapeutic outcomes and achieve the goal of curing dental caries. Improving efficacy and safety is a critical direction for future research. Based on a further exploration of the mechanisms of action of microbial preparations, refining and optimizing formulations according to research findings can enhance the therapeutic effects and safety of microbial preparations.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [32072184], the Natural Science Foundation of Guangdong Province [2023A1515011798], and the Research Start-up Foundation of Shantou University [NTF20003, NTF22003].
Author contributions
Si-Chen Luo: Conceptualization, Writing-Original draft preparation, Reviewing and Editing. Si-Min Wei: Search for literature, Writing-Reviewing and Editing. Xin-Tao Luo: Search for literature. Qiong-Qiong Yang: Writing-Editing. Ka-Hing Wong: Writing-Reviewing. Peter CK Cheung: Writing-Editing. Bo-Bo Zhang: Conceptualization, Supervision, and Writing-Reviewing and Editing.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wen PYF, Chen MX, Zhong YJ, Dong QQ, Wong HM. Global burden and inequality of dental caries, 1990 to 2019. J. Dent. Res. 2022;101:392–399. doi: 10.1177/00220345211056247. [DOI] [PubMed] [Google Scholar]
- 2.Nomura R, et al. Potential involvement of Streptococcus mutans possessing collagen binding protein Cnm in infective endocarditis. Sci. Rep. 2020;10:19118. doi: 10.1038/s41598-020-75933-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Philip N, Suneja B, Walsh LJ. Ecological approaches to dental caries prevention: paradigm shift or shibboleth? Caries Res. 2018;52:153–165. doi: 10.1159/000484985. [DOI] [PubMed] [Google Scholar]
- 4.Yu OY, Lam WY, Wong AW, Duangthip D, Chu CH. Nonrestorative management of dental caries. Dent. J. 2021;9:121. doi: 10.3390/dj9100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marsh PD, Head DA, Devine DA. Ecological approaches to oral biofilms: control without killing. Caries Res. 2015;49:46–54. doi: 10.1159/000377732. [DOI] [PubMed] [Google Scholar]
- 6.Meurman JH, Antila H, Salminen S. Recovery of Lactobacillus strain GG (ATCC 53103) from saliva of healthy volunteers after consumption of yoghurt prepared with the bacterium. Microb. Ecol. Health Dis. 1994;7:295–298. [Google Scholar]
- 7.Chattopadhyay I, et al. Can metagenomics unravel the impact of oral bacteriome in human diseases? Biotechnol. Genet. Eng. Rev. 2022;39:85–117. doi: 10.1080/02648725.2022.2102877. [DOI] [PubMed] [Google Scholar]
- 8.Achtman M, Zhou Z. Metagenomics of the modern and historical human oral microbiome with phylogenetic studies on Streptococcus mutans and Streptococcus sobrinus. Philos. Trans. R. Soc. B Biol. Sci. 2020;375:20190573. doi: 10.1098/rstb.2019.0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wade WG. The oral microbiome in health and disease. Pharmacol. Res. 2013;69:137–143. doi: 10.1016/j.phrs.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Rosier BT, Marsh PD, Mira A. Resilience of the oral microbiota in health: mechanisms that prevent dysbiosis. J. Dent. Res. 2018;97:371–380. doi: 10.1177/0022034517742139. [DOI] [PubMed] [Google Scholar]
- 11.Kilian M. The oral microbiome—friend or foe? Eur. J. Oral. Sci. 2018;126:5–12. doi: 10.1111/eos.12527. [DOI] [PubMed] [Google Scholar]
- 12.Kanasi E, et al. Clonal analysis of the microbiota of severe early childhood caries. Caries Res. 2010;44:485–497. doi: 10.1159/000320158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hajishengallis E, Parsaei Y, Klein MI, Koo H. Advances in the microbial etiology and pathogenesis of early childhood caries. Mol. Oral Microbiol. 2017;32:24–34. doi: 10.1111/omi.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forssten SD, Bjorklund M, Ouwehand AC. Streptococcus mutans, caries and simulation models. Nutrients. 2010;2:290–298. doi: 10.3390/nu2030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong Y, et al. Global transcriptional analysis of acid-inducible genes in Streptococcus mutans: multiple two-component systems involved in acid adaptation. Microbiology. 2009;155:3322–3332. doi: 10.1099/mic.0.031591-0. [DOI] [PubMed] [Google Scholar]
- 16.Kim D, et al. Spatial mapping of polymicrobial communities reveals a precise biogeography associated with human dental caries. Proc. Natl. Acad. Sci. USA. 2020;117:12375–12386. doi: 10.1073/pnas.1919099117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peres MA, et al. Oral diseases: a global public health challenge. Lancet. 2019;394:249–260. doi: 10.1016/S0140-6736(19)31146-8. [DOI] [PubMed] [Google Scholar]
- 18.Palmer RJ, et al. Interbacterial adhesion networks within early oral biofilms of single human hosts. Appl. Environ. Microbiol. 2017;83:e00407–e00417. doi: 10.1128/AEM.00407-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker JL, et al. Deep metagenomics examines the oral microbiome during dental caries, revealing novel taxa and co-occurrences with host molecules. Genome Res. 2021;31:64–74. doi: 10.1101/gr.265645.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu G, Wu C, Abrams WR, Li Y. Structural and functional characteristics of the microbiome in deep-dentin caries. J. Dent. Res. 2020;99:713–720. doi: 10.1177/0022034520913248. [DOI] [PubMed] [Google Scholar]
- 21.Jenkinson HF, Lamont RJ. Oral microbial communities in sickness and in health. Trends Microbiol. 2005;13:589–595. doi: 10.1016/j.tim.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Kazemtabrizi A, Haddadi A, Shavandi M, Harzandi N. Metagenomic investigation of bacteria associated with dental lesions: a cross-sectional study. Med. Oral Patol. Oral Cir. Bucal. 2020;25:e240–e251. doi: 10.4317/medoral.23326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterson SN, Snesrud E, Schork NJ, Bretz WA. Dental caries pathogenicity: a genomic and metagenomic perspective. Int. Dent. J. 2011;61:11–22. doi: 10.1111/j.1875-595X.2011.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 1997;10:505–520. doi: 10.1128/CMR.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sivamaruthi BS, Kesika P, Chaiyasut C. A review of the role of probiotic supplementation in dental caries. Probiotics Antimicrob. Proteins. 2020;12:1300–1309. doi: 10.1007/s12602-020-09652-9. [DOI] [PubMed] [Google Scholar]
- 26.He J, et al. RNA-Seq reveals enhanced sugar metabolism in Streptococcus mutans co-cultured with Candida albicans within mixed-species biofilms. Front. Microbiol. 2017;8:1036. doi: 10.3389/fmicb.2017.01036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Priya A, Selvaraj A, Divya D, Karthik Raja R, Pandian SK. In vitro and in vivo anti-infective potential of thymol against early childhood caries causing dual species Candida albicans and Streptococcus mutans. Front. Pharmacol. 2021;12:760768. doi: 10.3389/fphar.2021.760768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, et al. Core microbiota promotes the development of dental caries. Appl. Sci. 2021;11:3638. doi: 10.3390/app11083638. [DOI] [Google Scholar]
- 29.Belda-Ferre P, et al. The oral metagenome in health and disease. ISME J. 2012;6:46–56. doi: 10.1038/ismej.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pang L, et al. Metagenomic analysis of dental plaque on pit and fissure sites with and without caries among adolescents. Front. Cell. Infect. Microbiol. 2021;11:740981. doi: 10.3389/fcimb.2021.740981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Legenova K, Bujdakova H. The role of Streptococcus mutans in the oral biofilm. Epidemiol. Mikrobiol. Imunol. 2015;64:179–187. [PubMed] [Google Scholar]
- 33.Gross EL, et al. Bacterial 16S sequence analysis of severe caries in young permanent teeth. J. Clin. Microbiol. 2010;48:4121–4128. doi: 10.1128/JCM.01232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicolas GG, Lavoie MC. Streptococcus mutans and oral streptococci in dental plaque. Can. J. Microbiol. 2011;57:1–20. doi: 10.1139/W10-095. [DOI] [PubMed] [Google Scholar]
- 35.Balakrishnan M, Simmonds RS, Tagg JR. Dental caries is a preventable infectious disease. Aust. Dent. J. 2000;45:235–245. doi: 10.1111/j.1834-7819.2000.tb00257.x. [DOI] [PubMed] [Google Scholar]
- 36.Bowen WH. Rodent model in caries research. Odontology. 2013;101:9–14. doi: 10.1007/s10266-012-0091-0. [DOI] [PubMed] [Google Scholar]
- 37.Palmer CA, et al. Diet and caries-associated bacteria in severe early childhood caries. J. Dent. Res. 2010;89:1224–1229. doi: 10.1177/0022034510376543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin Y, Chen J, Zhou X, Li Y. Inhibition of Streptococcus mutans biofilm formation by strategies targeting the metabolism of exopolysaccharides. Crit. Rev. Microbiol. 2021;47:667–677. doi: 10.1080/1040841X.2021.1915959. [DOI] [PubMed] [Google Scholar]
- 39.Klein MI, Hwang G, Santos PHS, Campanella OH, Koo H. Streptococcus mutans-derived extracellular matrix in cariogenic oral biofilms. Front. Cell. Infect. Microbiol. 2015;5:10. doi: 10.3389/fcimb.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pleszczynska M, Wiater A, Janczarek M, Szczodrak J. (1->3)-α-D-glucan hydrolases in dental biofilm prevention and control: a review. Int. J. Biol. Macromol. 2015;79:761–778. doi: 10.1016/j.ijbiomac.2015.05.052. [DOI] [PubMed] [Google Scholar]
- 41.Poulin MB, Kuperman LL. Regulation of biofilm exopolysaccharide production by cyclic di-guanosine monophosphate. Front. Microbiol. 2021;12:730980. doi: 10.3389/fmicb.2021.730980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bowen WH, Burne RA, Wu H, Koo H. Oral biofilms: pathogens, matrix and polymicrobial interactions in microenvironments. Trends Microbiol. 2018;26:229–242. doi: 10.1016/j.tim.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alves LA, et al. CovR regulates Streptococcus mutans susceptibility to complement immunity and survival in blood. Infect. Immun. 2016;84:3206–3219. doi: 10.1128/IAI.00406-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodman SD, et al. Biofilms can be dispersed by focusing the immune system on a common family of bacterial nucleoid-associated proteins. Mucosal Immunol. 2011;4:625–637. doi: 10.1038/mi.2011.27. [DOI] [PubMed] [Google Scholar]
- 45.Xiao J, et al. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog. 2012;8:e1002623. doi: 10.1371/journal.ppat.1002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo L, McLean JS, Lux R, He X, Shi W. The well-coordinated linkage between acidogenicity and aciduricity via insoluble glucans on the surface of Streptococcus mutans. Sci. Rep. 2015;5:18015. doi: 10.1038/srep18015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Banas JA. Virulence properties of Streptococcus mutans. Front. Biosci. Landmark. 2004;9:1267–1277. doi: 10.2741/1305. [DOI] [PubMed] [Google Scholar]
- 48.Koo H, Allan RN, Howlin RP, Stoodley P, Hall-Stoodley L. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017;15:740–755. doi: 10.1038/nrmicro.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsumi Y, et al. Contribution of glucan-binding protein A to firm and stable biofilm formation by Streptococcus mutans. Mol. Oral Microbiol. 2015;30:217–226. doi: 10.1111/omi.12085. [DOI] [PubMed] [Google Scholar]
- 50.Abranches, J. et al. Biology of oral streptococci. Microbiol. Spectr. 6, 10.1128/microbiolspec.GPP3-0042-2018 (2018). [DOI] [PMC free article] [PubMed]
- 51.Xu X, Zhou XD, Wu CD. The tea catechin epigallocatechin gallate suppresses cariogenic virulence factors of Streptococcus mutans. Antimicrob. Agents Chemother. 2011;55:1229–1236. doi: 10.1128/AAC.01016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma Q, et al. Acetylation of lactate dehydrogenase negatively regulates the acidogenicity of Streptococcus mutans. mBio. 2022;13:e0201322. doi: 10.1128/mbio.02013-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cotter PD, Hill C. Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 2003;67:429–453. doi: 10.1128/MMBR.67.3.429-453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y-L, Nascimento M, Burne RA. Progress toward understanding the contribution of alkali generation in dental biofilms to inhibition of dental caries. Int. J. Oral Sci. 2012;4:135–140. doi: 10.1038/ijos.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li YH, Tian XL. Quorum sensing and bacterial social interactions in biofilms. Sensors. 2012;12:2519–2538. doi: 10.3390/s120302519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsumoto-Nakano M. Role of Streptococcus mutans surface proteins for biofilm formation. Jpn. Dent. Sci. Rev. 2018;54:22–29. doi: 10.1016/j.jdsr.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lei L, et al. Modulation of biofilm exopolysaccharides by the Streptococcus mutans vicX gene. Front. Microbiol. 2015;6:1432. doi: 10.3389/fmicb.2015.01432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sadeghinejad L, et al. Mechanistic, genomic and proteomic study on the effects of BisGMA-derived biodegradation product on cariogenic bacteria. Dent. Mater. 2017;33:175–190. doi: 10.1016/j.dental.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woelber JP, Al-Ahmad A, Alt KW. On the pathogenicity of the oral biofilm: a critical review from a biological, evolutionary, and nutritional point of view. Nutrients. 2022;14:2174. doi: 10.3390/nu14102174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dashiff A, Kadouri DE. Predation of oral pathogens by Bdellovibrio bacteriovorus 109J. Mol. Oral Microbiol. 2011;26:19–34. doi: 10.1111/j.2041-1014.2010.00592.x. [DOI] [PubMed] [Google Scholar]
- 61.Van Essche M, et al. Killing of anaerobic pathogens by predatory bacteria. Mol. Oral Microbiol. 2011;26:52–61. doi: 10.1111/j.2041-1014.2010.00595.x. [DOI] [PubMed] [Google Scholar]
- 62.Zarco MF, Vess TJ, Ginsburg GS. The oral microbiome in health and disease and the potential impact on personalized dental medicine. Oral Dis. 2012;18:109–120. doi: 10.1111/j.1601-0825.2011.01851.x. [DOI] [PubMed] [Google Scholar]
- 63.Mercenier A, Pavan S, Pot B. Probiotics as biotherapeutic agents: present knowledge and future prospects. Curr. Pharm. Des. 2003;9:175. doi: 10.2174/1381612033392224. [DOI] [PubMed] [Google Scholar]
- 64.Hill C, et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 65.Saiz P, Taveira N, Alves R. Probiotics in oral health and disease: a systematic review. Appl. Sci. 2021;11:8070. doi: 10.3390/app11178070. [DOI] [Google Scholar]
- 66.Simark-Mattsson C, et al. Lactobacillus-mediated interference of mutans streptococci in caries-free vs. caries-active subjects. Eur. J. Oral Sci. 2007;115:308–314. doi: 10.1111/j.1600-0722.2007.00458.x. [DOI] [PubMed] [Google Scholar]
- 67.Inchingolo AD, et al. Oralbiotica/oralbiotics: the impact of oral microbiota on dental health and demineralization: a systematic review of the literature. Children. 2022;9:1014. doi: 10.3390/children9071014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Teughels W, Van Essche M, Sliepen I, Quirynen M. Probiotics and oral healthcare. Periodontology. 2008;48:111–147. doi: 10.1111/j.1600-0757.2008.00254.x. [DOI] [PubMed] [Google Scholar]
- 69.Talarico TL, Casas IA, Chung TC, Dobrogosz WJ. Production and isolation of reuterin, a growth inhibitor produced by Lactobacillus reuteri. Antimicrob. Agents Chemother. 1988;32:1854–1858. doi: 10.1128/AAC.32.12.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gänzle MG, Höltzel A, Walter J, Jung G, Hammes WP. Characterization of reutericyclin produced by Lactobacillus reuteri LTH2584. Appl. Environ. Microbiol. 2000;66:4325–4333. doi: 10.1128/AEM.66.10.4325-4333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Caglar E, et al. Effect of yogurt with Bifidobacterium DN-173 010 on salivary mutans streptococci and lactobacilli in young adults. Acta Odontol. Scand. 2005;63:317–320. doi: 10.1080/00016350510020070. [DOI] [PubMed] [Google Scholar]
- 72.Darbandi A, et al. Bacteriocins: properties and potential use as antimicrobials. J. Clin. Lab. Anal. 2022;36:e24093. doi: 10.1002/jcla.24093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rogers LA. The inhibiting effect of Streptococcus lactis on Lactobacillus bulgaricus. J. Bacteriol. 1928;16:321–325. doi: 10.1128/jb.16.5.321-325.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heng BC. Reluctance of medical professionals in adopting natural-cycle and minimal ovarian stimulation protocols in human clinical assisted reproduction. Reprod. Biomed. Online. 2007;15:9–11. doi: 10.1016/S1472-6483(10)60683-9. [DOI] [PubMed] [Google Scholar]
- 75.Wang Y, Qin Y, Zhang Y, Wu R, Li P. Antibacterial mechanism of plantaricin LPL-1, a novel class IIa bacteriocin against Listeria monocytogenes. Food Control. 2019;97:87–93. doi: 10.1016/j.foodcont.2018.10.025. [DOI] [Google Scholar]
- 76.Surachat K, Sangket U, Deachamag P, Chotigeat W. In silico analysis of protein toxin and bacteriocins from Lactobacillus paracasei SD1 genome and available online databases. PLoS One. 2017;12:e0183548. doi: 10.1371/journal.pone.0183548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nagao J, et al. Lantibiotics: insight and foresight for new paradigm. J. Biosci. Bioeng. 2006;102:139–149. doi: 10.1263/jbb.102.139. [DOI] [PubMed] [Google Scholar]
- 78.Yang S-C, Lin C-H, Sung CT, Fang J-Y. Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Front. Microbiol. 2014;5:241. doi: 10.3389/fmicb.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jin X, An S, Kightlinger W, Zhou J, Hong SH. Engineering Escherichia coli to produce and secrete colicins for rapid and selective biofilm cell killing. AIChE J. 2021;67:e17466. doi: 10.1002/aic.17466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dobson A, Cotter PD, Ross RP, Hill C. Bacteriocin production: a probiotic trait? Appl. Environ. Microbiol. 2012;78:1–6. doi: 10.1128/AEM.05576-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Radaic A, et al. Modulation of pathogenic oral biofilms towards health with nisin probiotic. J. Oral. Microbiol. 2020;12:1809302. doi: 10.1080/20002297.2020.1809302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Conrads G, Westenberger J, Luerkens M, Abdelbary MMH. Isolation and bacteriocin-related typing of Streptococcus dentisani. Front. Cell Infect. Microbiol. 2019;9:110. doi: 10.3389/fcimb.2019.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jaffar N, Ishikawa Y, Mizuno K, Okinaga T, Maeda T. Mature biofilm degradation by potential probiotics: Aggregatibacter actinomycetemcomitans versus Lactobacillus spp. PLoS One. 2016;11:e0159466. doi: 10.1371/journal.pone.0159466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Walker GV, et al. Salivaricin E and abundant dextranase activity may contribute to the anti-cariogenic potential of the probiotic candidate Streptococcus salivarius JH. Microbiology. 2016;162:476–486. doi: 10.1099/mic.0.000237. [DOI] [PubMed] [Google Scholar]
- 85.Huang X, et al. A highly arginolytic Streptococcus species that potently antagonizes Streptococcus mutans. Appl. Environ. Microbiol. 2016;82:2187–2201. doi: 10.1128/AEM.03887-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Di Pierro F, Zanvit A, Nobili P, Risso P, Fornaini C. Cariogram outcome after 90 days of oral treatment with Streptococcus salivarius M18 in children at high risk for dental caries: results of a randomized, controlled study. Clin. Cosmet. Investig. Dent. 2015;7:107–113. doi: 10.2147/CCIDE.S93066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Satpute SK, et al. Biosurfactant/s from lactobacilli species: properties, challenges and potential biomedical applications. J. Basic Microbiol. 2016;56:1140–1158. doi: 10.1002/jobm.201600143. [DOI] [PubMed] [Google Scholar]
- 88.Sharma D, Singh Saharan B. Simultaneous production of biosurfactants and bacteriocins by probiotic Lactobacillus casei MRTL3. Int. J. Microbiol. 2014;2014:698713. doi: 10.1155/2014/698713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rodrigues LR, Teixeira JA, Oliveira R. Low-cost fermentative medium for biosurfactant production by probiotic bacteria. Biochem. Eng. J. 2006;32:135–142. doi: 10.1016/j.bej.2006.09.012. [DOI] [Google Scholar]
- 90.Saravanakumari P, Mani K. Structural characterization of a novel xylolipid biosurfactant from Lactococcus lactis and analysis of antibacterial activity against multi-drug resistant pathogens. Bioresour. Technol. 2010;101:8851–8854. doi: 10.1016/j.biortech.2010.06.104. [DOI] [PubMed] [Google Scholar]
- 91.Thavasi R, Jayalakshmi S, Banat IM. Effect of biosurfactant and fertilizer on biodegradation of crude oil by marine isolates of Bacillus megaterium, Corynebacterium kutscheri and Pseudomonas aeruginosa. Bioresour. Technol. 2011;102:772–778. doi: 10.1016/j.biortech.2010.08.099. [DOI] [PubMed] [Google Scholar]
- 92.Ciandrini E, et al. Characterization of biosurfactants produced by Lactobacillus spp. and their activity against oral streptococci biofilm. Appl. Microbiol. Biotechnol. 2016;100:6767–6777. doi: 10.1007/s00253-016-7531-7. [DOI] [PubMed] [Google Scholar]
- 93.Tahmourespour A, Salehi R, Kasra Kermanshahi R. Lactobacillus acidophilus-derived biosurfactant effect on gtfB and gtfC expression level in Streptococcus mutans biofilm cells. Braz. J. Microbiol. 2011;42:330–339. doi: 10.1590/S1517-83822011000100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tan Y, Leonhard M, Moser D, Schneider-Stickler B. Inhibition activity of Lactobacilli supernatant against fungal-bacterial multispecies biofilms on silicone. Microb. Pathog. 2017;113:197–201. doi: 10.1016/j.micpath.2017.10.051. [DOI] [PubMed] [Google Scholar]
- 95.Gudina EJ, Teixeira JA, Rodrigues LR. Isolation and functional characterization of a biosurfactant produced by Lactobacillus paracasei. Colloids Surf. B Biointerfaces. 2010;76:298–304. doi: 10.1016/j.colsurfb.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 96.Özcelik S, Kuley E, Özogul F. Formation of lactic, acetic, succinic, propionic, formic and butyric acid by lactic acid bacteria. LWT Food Sci. Technol. 2016;73:536–542. doi: 10.1016/j.lwt.2016.06.066. [DOI] [Google Scholar]
- 97.Lin X, Chen X, Chen Y, Jiang W, Chen H. The effect of five probiotic lactobacilli strains on the growth and biofilm formation of Streptococcus mutans. Oral Dis. 2015;21:E128–E134. doi: 10.1111/odi.12257. [DOI] [PubMed] [Google Scholar]
- 98.Bustamante M, Oomah BD, Mosi-Roa Y, Rubilar M, Burgos-Diaz C. Probiotics as an adjunct therapy for the treatment of halitosis, dental caries and periodontitis. Probiotics Antimicrob. Proteins. 2020;12:325–334. doi: 10.1007/s12602-019-9521-4. [DOI] [PubMed] [Google Scholar]
- 99.Redanz S, et al. Live and let die: hydrogen peroxide production by the commensal flora and its role in maintaining a symbiotic microbiome. Mol. Oral Microbiol. 2018;33:337–352. doi: 10.1111/omi.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Herrero ER, et al. Antimicrobial effects of commensal oral species are regulated by environmental factors. J. Dent. 2016;47:23–33. doi: 10.1016/j.jdent.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 101.El Oirdi S, et al. Isolation and identification of Lactobacillus plantarum 4F, a strain with high antifungal activity, fungicidal effect, and biopreservation properties of food. J. Food Process. Preserv. 2021;45:e15517. doi: 10.1111/jfpp.15517. [DOI] [Google Scholar]
- 102.Lai W-K, et al. Developing lactic acid bacteria as an oral healthy food. Life. 2021;11:268. doi: 10.3390/life11040268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barzegari A, et al. The battle of probiotics and their derivatives against biofilms. Infect. Drug Resist. 2020;13:659–672. doi: 10.2147/IDR.S232982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wasfi R, Abd El-Rahman OA, Zafer MM, Ashour HM. Probiotic Lactobacillus sp. inhibit growth, biofilm formation and gene expression of caries-inducing Streptococcus mutans. J. Cell. Mol. Med. 2018;22:1972–1983. doi: 10.1111/jcmm.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Matsubara VH, Wang Y, Bandara HMHN, Mayer MPA, Samaranayake LP. Probiotic lactobacilli inhibit early stages of Candida albicans biofilm development by reducing their growth, cell adhesion, and filamentation. Appl. Microbiol. Biotechnol. 2016;100:6415–6426. doi: 10.1007/s00253-016-7527-3. [DOI] [PubMed] [Google Scholar]
- 106.James KM, MacDonald KW, Chanyi RM, Cadieux PA, Burton JP. Inhibition of Candida albicans biofilm formation and modulation of gene expression by probiotic cells and supernatant. J. Med. Microbiol. 2016;65:328–336. doi: 10.1099/jmm.0.000226. [DOI] [PubMed] [Google Scholar]
- 107.Cortes-Acha B, et al. Development and viability of biofilms grown on experimental abutments mimicking dental implants: an in vivo model. Med. Oral Patol. Oral. Cir. Bucal. 2019;24:e511–e517. doi: 10.4317/medoral.22868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jung H-Y, et al. Collagen peptide in a combinatorial treatment with Lactobacillus rhamnosus inhibits the cariogenic properties of Streptococcus mutans: an in vitro study. Int. J. Mol. Sci. 2022;23:1860. doi: 10.3390/ijms23031860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lin T-H, Lin C-H, Pan T-M. The implication of probiotics in the prevention of dental caries. Appl. Microbiol. Biotechnol. 2018;102:577–586. doi: 10.1007/s00253-017-8664-z. [DOI] [PubMed] [Google Scholar]
- 110.Singh TP, Kaur G, Kapila S, Malik RK. Antagonistic activity of Lactobacillus reuteri strains on the adhesion characteristics of selected pathogens. Front. Microbiol. 2017;8:486. doi: 10.3389/fmicb.2017.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Burton JP, et al. Influence of the probiotic Streptococcus salivarius strain M18 on indices of dental health in children: a randomized double-blind, placebo-controlled trial. J. Med. Microbiol. 2013;62:875–884. doi: 10.1099/jmm.0.056663-0. [DOI] [PubMed] [Google Scholar]
- 112.Ha Kim J, Jang HJ, Lee N-K, Paik H-D. Antibacterial and antibiofilm effect of cell-free supernatant of Lactobacillus brevis KCCM 202399 isolated from korean fermented food against Streptococcus mutans KCTC 5458. J. Microbiol. Biotechnol. 2022;32:56–63. doi: 10.4014/jmb.2109.09045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Haukioja A, Loimaranta V, Tenovuo J. Probiotic bacteria affect the composition of salivary pellicle and streptococcal adhesion in vitro. Oral Microbiol. Immunol. 2008;23:336–343. doi: 10.1111/j.1399-302X.2008.00435.x. [DOI] [PubMed] [Google Scholar]
- 114.Tenovuo J. Antimicrobial function of human saliva-how important is it for oral health? Acta Odontol. Scand. 1998;56:250–256. doi: 10.1080/000163598428400. [DOI] [PubMed] [Google Scholar]
- 115.Boris S, Suárez JE, Barbés C. Characterization of the aggregation promoting factor from Lactobacillus gasseri, a vaginal isolate. J. Appl. Microbiol. 1997;83:413–420. doi: 10.1046/j.1365-2672.1997.00250.x. [DOI] [PubMed] [Google Scholar]
- 116.Lang C, et al. Specific Lactobacillus/mutans Streptococcus co-aggregation. J. Dent. Res. 2010;89:175–179. doi: 10.1177/0022034509356246. [DOI] [PubMed] [Google Scholar]
- 117.Sliepen I, et al. Microbial interactions influence inflammatory host cell responses. J. Dent. Res. 2009;88:1026–1030. doi: 10.1177/0022034509347296. [DOI] [PubMed] [Google Scholar]
- 118.Wattanarat O, et al. Significant elevation of salivary human neutrophil peptides 1-3 levels by probiotic milk in preschool children with severe early childhood caries: a randomized controlled trial. Clin. Oral Investig. 2021;25:2891–2903. doi: 10.1007/s00784-020-03606-9. [DOI] [PubMed] [Google Scholar]
- 119.Pahumunto N, Sophatha B, Piwat S, Teanpaisan R. Increasing salivary IgA and reducing Streptococcus mutans by probiotic Lactobacillus paracasei SD1: a double-blind, randomized, controlled study. J. Dent. Sci. 2019;14:178–184. doi: 10.1016/j.jds.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Balzaretti S, et al. A novel rhamnose-rich hetero-exopolysaccharide isolated from Lactobacillus paracasei DG activates THP-1 human monocytic cells. Appl. Environ. Microbiol. 2017;83:e02702–e02716. doi: 10.1128/AEM.02702-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Amargianitakis M, Antoniadou M, Rahiotis C, Varzakas T. Probiotics, prebiotics, synbiotics and dental caries. new perspectives, suggestions, and patient coaching approach for a cavity-free mouth. Appl. Sci. 2021;11:5472. doi: 10.3390/app11125472. [DOI] [Google Scholar]
- 122.Nadelman P, Magno MB, Masterson D, da Cruz AG, Maia LC. Are dairy products containing probiotics beneficial for oral health? a systematic review and meta-analysis. Clin. Oral Investig. 2018;22:2763–2785. doi: 10.1007/s00784-018-2682-9. [DOI] [PubMed] [Google Scholar]
- 123.Gedalia I, et al. Enamel softening with Coca-Cola and rehardening with milk or saliva. Am. J. Dent. 1991;4:120–122. [PubMed] [Google Scholar]
- 124.Kashket S, Yaskell T. Effectiveness of calcium lactate added to food in reducing intraoral demineralization of enamel. Caries Res. 1997;31:429–433. doi: 10.1159/000262434. [DOI] [PubMed] [Google Scholar]
- 125.Schüpbach P, Neeser JR, Golliard M, Rouvet M, Guggenheim B. Incorporation of caseinoglycomacropeptide and caseinophosphopeptide into the salivary pellicle inhibits adherence of mutans streptococci. J. Dent. Res. 1996;75:1779–1788. doi: 10.1177/00220345960750101101. [DOI] [PubMed] [Google Scholar]
- 126.Swarna SK, Nivedhitha MS. Probiotics in prevention of dental caries—a literature review. Biosci. Biotechnol. Res. Commun. 2020;13:517–526. doi: 10.21786/bbrc/13.8/190. [DOI] [Google Scholar]
- 127.de Alvarenga JA, et al. Probiotic effects of lactobacillus paracasei 28.4 to inhibit Streptococcus mutans in a gellan-based formulation. Probiotics Antimicrob. Proteins. 2021;13:506–517. doi: 10.1007/s12602-020-09712-0. [DOI] [PubMed] [Google Scholar]
- 128.Yelin I, et al. Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat. Med. 2019;25:1728–1732. doi: 10.1038/s41591-019-0626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gruner D, Paris S, Schwendicke F. Probiotics for managing caries and periodontitis: systematic review and meta-analysis. J. Dent. 2016;48:16–25. doi: 10.1016/j.jdent.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 130.Corby PM, et al. Microbial risk indicators of early childhood caries. J. Clin. Microbiol. 2005;43:5753–5759. doi: 10.1128/JCM.43.11.5753-5759.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wen ZT, Huang X, Ellepola K, Liao S, Li Y. Lactobacilli and human dental caries: more than mechanical retention. Microbiology. 2022;168:001196. doi: 10.1099/mic.0.001196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Henne K, Rheinberg A, Melzer-Krick B, Conrads G. Aciduric microbial taxa including Scardovia wiggsiae and Bifidobacterium spp. in caries and caries free subjects. Anaerobe. 2015;35:60–65. doi: 10.1016/j.anaerobe.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 133.Caufield PW, Schön CN, Saraithong P, Li Y, Argimón S. Oral lactobacilli and dental caries: a model for niche adaptation in humans. J. Dent. Res. 2015;94:110S–118S. doi: 10.1177/0022034515576052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Newhouse MT, Dolovich M. Spacer devices for asthma. J. Pediatr. 1986;109:913–914. doi: 10.1016/S0022-3476(86)80736-3. [DOI] [PubMed] [Google Scholar]
- 135.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 136.Gibson GR, et al. Expert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 137.Guerrero-Wyss M, Durán Agüero S, Angarita Dávila L. D-tagatose is a promising sweetener to control glycaemia: a new functional food. Biomed. Res. Int. 2018;2018:e8718053. doi: 10.1155/2018/8718053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mayumi S, et al. Potential of prebiotic D-tagatose for prevention of oral disease. Front. Cell Infect. Microbiol. 2021;11:767944. doi: 10.3389/fcimb.2021.767944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nagamine Y, et al. D-tagatose effectively reduces the number of Streptococcus mutans and oral bacteria in healthy adult subjects: a chewing gum pilot study and randomized clinical trial. Acta Med. Okayama. 2020;74:307–317. doi: 10.18926/AMO/60369. [DOI] [PubMed] [Google Scholar]
- 140.Kojima Y, Ohshima T, Seneviratne CJ, Maeda N. Combining prebiotics and probiotics to develop novel synbiotics that suppress oral pathogens. J. Oral Biosci. 2016;58:27–32. doi: 10.1016/j.job.2015.08.004. [DOI] [Google Scholar]
- 141.Söderling E, Pienihäkkinen K. Effects of xylitol and erythritol consumption on mutans streptococci and the oral microbiota: a systematic review. Acta Odontol. Scand. 2020;78:599–608. doi: 10.1080/00016357.2020.1788721. [DOI] [PubMed] [Google Scholar]
- 142.Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr. Res. Rev. 2004;17:259–275. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- 143.Roberfroid M, et al. Prebiotic effects: metabolic and health benefits. Br. J. Nutr. 2010;104:S1–S63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 144.Cocco F, et al. The caries preventive effect of 1-year use of low-dose xylitol chewing gum. a randomized placebo-controlled clinical trial in high-caries-risk adults. Clin. Oral Investig. 2017;21:2733–2740. doi: 10.1007/s00784-017-2075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Söderling E, Alaräisänen L, Scheinin A, Mäkinen KK. Effect of xylitol and sorbitol on polysaccharide production by and adhesive properties of Streptococcus mutans. Caries Res. 1987;21:109–116. doi: 10.1159/000261011. [DOI] [PubMed] [Google Scholar]
- 146.Watthanasaen S, et al. Xylitol-containing chewing gum for caries prevention in students with disabilities: a randomised trial. Oral Health Prev. Dent. 2017;15:519–527. doi: 10.3290/j.ohpd.a39668. [DOI] [PubMed] [Google Scholar]
- 147.Gauthier L, Vadeboncoeur C, Mayrand D. Loss of sensitivity to xylitol by Streptococcus mutans LG-1. Caries Res. 1984;18:289–295. doi: 10.1159/000260779. [DOI] [PubMed] [Google Scholar]
- 148.Falony G, et al. Long-term effect of erythritol on dental caries development during childhood: a posttreatment survival analysis. Caries Res. 2016;50:579–588. doi: 10.1159/000450762. [DOI] [PubMed] [Google Scholar]
- 149.Thabuis C, et al. Effects of maltitol and xylitol chewing-gums on parameters involved in dental caries development. Eur. J. Paediatr. Dent. 2013;14:303–308. [PubMed] [Google Scholar]
- 150.Salli K, Söderling E, Hirvonen J, Gürsoy UK, Ouwehand AC. Influence of 2′-fucosyllactose and galacto-oligosaccharides on the growth and adhesion of Streptococcus mutans. Br. J. Nutr. 2020;124:824–831. doi: 10.1017/S0007114520001956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sharon N. Carbohydrates as future anti-adhesion drugs for infectious diseases. Biochim. Biophys. Acta. 2006;1760:527–537. doi: 10.1016/j.bbagen.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 152.Oku T, Nakamura S. Threshold for transitory diarrhea induced by ingestion of xylitol and lactitol in young male and female adults. J. Nutr. Sci. Vitaminol. 2007;53:13–20. doi: 10.3177/jnsv.53.13. [DOI] [PubMed] [Google Scholar]
- 153.Koopman JE, et al. Stability and resilience of oral microcosms toward acidification and Candida outgrowth by arginine supplementation. Microb. Ecol. 2015;69:422–433. doi: 10.1007/s00248-014-0535-x. [DOI] [PubMed] [Google Scholar]
- 154.Bacali C, et al. Oral microbiome: getting to know and befriend neighbors, a biological approach. Biomedicines. 2022;10:671. doi: 10.3390/biomedicines10030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zheng X, et al. Ecological effect of arginine on oral microbiota. Sci. Rep. 2017;7:7206. doi: 10.1038/s41598-017-07042-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.He J, et al. L-arginine modifies the exopolysaccharide matrix and thwarts Streptococcus mutans outgrowth within mixed-species oral biofilms. J. Bacteriol. 2016;198:2651–2661. doi: 10.1128/JB.00021-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Koopman JE, et al. Changes in the oral ecosystem induced by the use of 8% arginine toothpaste. Arch. Oral Biol. 2017;73:79–87. doi: 10.1016/j.archoralbio.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 158.Yin W, et al. The anti-caries efficacy of a dentifrice containing 1.5% arginine and 1450 ppm fluoride as sodium monofluorophosphate assessed using quantitative light-induced fluorescence (QLF) J. Dent. 2013;41:S22–S28. doi: 10.1016/j.jdent.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 159.Bijle MNA, Ekambaram M, Lo EC, Yiu CKY. The combined enamel remineralization potential of arginine and fluoride toothpaste. J. Dent. 2018;76:75–82. doi: 10.1016/j.jdent.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 160.Carda-Diéguez M, Moazzez R, Mira A. Functional changes in the oral microbiome after use of fluoride and arginine containing dentifrices: a metagenomic and metatranscriptomic study. Microbiome. 2022;10:159. doi: 10.1186/s40168-022-01338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cheng X, et al. Magnesium-dependent promotion of H2O2 production increases ecological competitiveness of oral commensal streptococci. J. Dent. Res. 2020;99:847–854. doi: 10.1177/0022034520912181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Burne RA, Marquis RE. Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol. Lett. 2000;193:1–6. doi: 10.1111/j.1574-6968.2000.tb09393.x. [DOI] [PubMed] [Google Scholar]
- 163.Zaura E, Twetman S. Critical appraisal of oral pre- and probiotics for caries prevention and care. Caries Res. 2019;53:514–526. doi: 10.1159/000499037. [DOI] [PubMed] [Google Scholar]
- 164.Sánchez GA, Miozza VA, Delgado A, Busch L. Total salivary nitrates and nitrites in oral health and periodontal disease. Nitric Oxide. 2014;36:31–35. doi: 10.1016/j.niox.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 165.Doel JJ, et al. Protective effect of salivary nitrate and microbial nitrate reductase activity against caries. Eur. J. Oral Sci. 2004;112:424–428. doi: 10.1111/j.1600-0722.2004.00153.x. [DOI] [PubMed] [Google Scholar]
- 166.Green SJ. Nitric oxide in mucosal immunity. Nat. Med. 1995;1:515–517. doi: 10.1038/nm0695-515. [DOI] [PubMed] [Google Scholar]
- 167.Allaker RP, Silva Mendez LS, Hardie JM, Benjamin N. Antimicrobial effect of acidified nitrite on periodontal bacteria. Oral Microbiol. Immunol. 2001;16:253–256. doi: 10.1034/j.1399-302X.2001.160410.x. [DOI] [PubMed] [Google Scholar]
- 168.Rosier BT, Buetas E, Moya-Gonzalvez EM, Artacho A, Mira A. Nitrate as a potential prebiotic for the oral microbiome. Sci. Rep. 2020;10:12895. doi: 10.1038/s41598-020-69931-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li H, et al. Salivary nitrate—an ecological factor in reducing oral acidity. Oral Microbiol. Immunol. 2007;22:67–71. doi: 10.1111/j.1399-302X.2007.00313.x. [DOI] [PubMed] [Google Scholar]
- 170.Jockel-Schneider Y, et al. Stimulation of the nitrate-nitrite-NO-metabolism by repeated lettuce juice consumption decreases gingival inflammation in periodontal recall patients: a randomized, double-blinded, placebo-controlled clinical trial. J. Clin. Periodontol. 2016;43:603–608. doi: 10.1111/jcpe.12542. [DOI] [PubMed] [Google Scholar]
- 171.Gee LC, Ahluwalia A. Dietary nitrate lowers blood pressure: epidemiological, pre-clinical experimental and clinical trial evidence. Curr. Hypertens. Rep. 2016;18:17. doi: 10.1007/s11906-015-0623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Vanhatalo A, et al. Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free Radic. Biol. Med. 2018;124:21–30. doi: 10.1016/j.freeradbiomed.2018.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Velmurugan S, et al. Dietary nitrate improves vascular function in patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 2015;103:25–38. doi: 10.3945/ajcn.115.116244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Markowiak P, Śliżewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9:1021. doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Swanson KS, et al. The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020;17:687–701. doi: 10.1038/s41575-020-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nunpan S, Suwannachart C, Wayakanon K. Effect of prebiotics-enhanced probiotics on the growth of Streptococcus mutans. Int. J. Microbiol. 2019;2019:4623807. doi: 10.1155/2019/4623807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tester R, Al-Ghazzewi F. A preliminary study of the synbiotic effects of konjac glucomannan hydrolysates (GMH) and lactobacilli on the growth of the oral bacterium Streptococcus mutans. Nutr. Food Sci. 2011;41:234–237. doi: 10.1108/00346651111151357. [DOI] [Google Scholar]
- 178.Bijle MN, Neelakantan P, Ekambaram M, Lo ECM, Yiu CKY. Effect of a novel synbiotic on Streptococcus mutans. Sci. Rep. 2020;10:7951. doi: 10.1038/s41598-020-64956-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Salminen S, et al. The international scientific association of probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021;18:649–667. doi: 10.1038/s41575-021-00440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Barros CP, et al. Paraprobiotics and postbiotics: concepts and potential applications in dairy products. Curr. Opin. Food Sci. 2020;32:1–8. doi: 10.1016/j.cofs.2019.12.003. [DOI] [Google Scholar]
- 181.Moradi M, et al. Postbiotics produced by lactic acid bacteria: the next frontier in food safety. Compr. Rev. Food Sci. Food Saf. 2020;19:3390–3415. doi: 10.1111/1541-4337.12613. [DOI] [PubMed] [Google Scholar]
- 182.Holz C, et al. Lactobacillus paracasei DSMZ16671 reduces mutans Streptococci: a short-term pilot study. Probiotics Antimicrob. Proteins. 2013;5:259–263. doi: 10.1007/s12602-013-9148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Moradi M, Molaei R, Guimarães JT. A review on preparation and chemical analysis of postbiotics from lactic acid bacteria. Enzym. Microb. Technol. 2021;143:109722. doi: 10.1016/j.enzmictec.2020.109722. [DOI] [PubMed] [Google Scholar]
- 184.el-Nezami H, Kankaanpää P, Salminen S, Ahokas J. Physicochemical alterations enhance the ability of dairy strains of lactic acid bacteria to remove aflatoxin from contaminated media. J. Food Prot. 1998;61:466–468. doi: 10.4315/0362-028X-61.4.466. [DOI] [PubMed] [Google Scholar]
- 185.Schwendicke F, Horb K, Kneist S, Dörfer C, Paris S. Effects of heat-inactivated Bifidobacterium BB12 on cariogenicity of Streptococcus mutans in vitro. Arch. Oral Biol. 2014;59:1384–1390. doi: 10.1016/j.archoralbio.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 186.Tareb R, Bernardeau M, Gueguen M, Vernoux J-P. In vitro characterization of aggregation and adhesion properties of viable and heat-killed forms of two probiotic Lactobacillus strains and interaction with foodborne zoonotic bacteria, especially Campylobacter jejuni. J. Med. Microbiol. 2013;62:637–649. doi: 10.1099/jmm.0.049965-0. [DOI] [PubMed] [Google Scholar]
- 187.Pahumunto N, et al. Reducing mutans streptococci and caries development by Lactobacillus paracasei SD1 in preschool children: a randomized placebo-controlled trial. Acta Odontol. Scand. 2018;76:331–337. doi: 10.1080/00016357.2018.1453083. [DOI] [PubMed] [Google Scholar]
- 188.Ritthagol W, Saetang C, Teanpaisan R. Effect of probiotics containing Lactobacillus paracasei SD1 on salivary mutans streptococci and lactobacilli in orthodontic cleft patients: a double-blinded, randomized, placebo-controlled study. Cleft Palate Craniofac. J. 2014;51:257–263. doi: 10.1597/12-243. [DOI] [PubMed] [Google Scholar]
- 189.Nozari A, Motamedifar M, Seifi N, Hatamizargaran Z, Ranjbar MA. The effect of Iranian customary used probiotic yogurt on the children’s salivary cariogenic microflora. J. Dent. 2015;16:81–86. [PMC free article] [PubMed] [Google Scholar]
- 190.Pinto GS, Cenci MS, Azevedo MS, Epifanio M, Jones MH. Effect of yogurt containing Bifidobacterium animalis subsp. lactis DN-173010 probiotic on dental plaque and saliva in orthodontic patients. Caries Res. 2014;48:63–68. doi: 10.1159/000353467. [DOI] [PubMed] [Google Scholar]
- 191.Zare Javid A, et al. Effects of the consumption of probiotic yogurt containing Bifidobacterium lactis Bb12 on the levels of Streptococcus mutans and lactobacilli in saliva of students with initial stages of dental caries: a double-blind randomized controlled trial. Caries Res. 2020;54:68–74. doi: 10.1159/000504164. [DOI] [PubMed] [Google Scholar]
- 192.Miyazima T, Ishikawa K, Mayer M, Saad S, Nakamae A. Cheese supplemented with probiotics reduced the Candida levels in denture wearers—RCT. Oral Dis. 2017;23:919–925. doi: 10.1111/odi.12669. [DOI] [PubMed] [Google Scholar]
- 193.Ahola AJ, et al. Short-term consumption of probiotic-containing cheese and its effect on dental caries risk factors. Arch. Oral Biol. 2002;47:799–804. doi: 10.1016/S0003-9969(02)00112-7. [DOI] [PubMed] [Google Scholar]
- 194.Mortazavi S, Akhlaghi N. Salivary Streptococcus mutans and Lactobacilli levels following probiotic cheese consumption in adults: a double blind randomized clinical trial*. J. Res. Med. Sci. 2012;17:57–66. [PMC free article] [PubMed] [Google Scholar]
- 195.Ashwin D, et al. Effect of probiotic containing ice-cream on salivary mutans streptococci (SMS) levels in children of 6-12 years of age: a randomized controlled double blind study with six-months follow up. J. Clin. Diagn. Res. 2015;9:ZC06–ZC09. doi: 10.7860/JCDR/2015/10942.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hasslof P, West CE, Videhult FK, Brandelius C, Stecksen-Blicks C. Early intervention with probiotic Lactobacillus paracasei F19 has no long-term effect on caries experience. Caries Res. 2013;47:559–565. doi: 10.1159/000350524. [DOI] [PubMed] [Google Scholar]
- 197.Taipale T, Pienihakkinen K, Salminen S, Jokela J, Soderling E. Bifidobacterium animalis subsp. lactis BB-12 administration in early childhood: a randomized clinical trial of effects on oral colonization by mutans streptococci and the probiotic. Caries Res. 2012;46:69–77. doi: 10.1159/000335567. [DOI] [PubMed] [Google Scholar]
- 198.Caglar E, et al. Effect of chewing gums containing xylitol or probiotic bacteria on salivary mutans streptococci and lactobacilli. Clin. Oral Investig. 2007;11:425–429. doi: 10.1007/s00784-007-0129-9. [DOI] [PubMed] [Google Scholar]
- 199.Srivastava S, Saha S, Kumari M, Mohd S. Effect of probiotic curd on salivary pH and Streptococcus mutans: a double blind parallel randomized controlled trial. J. Clin. Diagn. Res. 2016;10:ZC13–ZC16. doi: 10.7860/JCDR/2016/15530.7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Jose JE, Padmanabhan S, Chitharanjan AB. Systemic consumption of probiotic curd and use of probiotic toothpaste to reduce Streptococcus mutans in plaque around orthodontic brackets. Am. J. Orthod. Dentofac. Orthop. 2013;144:67–72. doi: 10.1016/j.ajodo.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 201.Pohjavuori S, et al. Effect of consumption of Lactobacillus rhamnosus GG and calcium, in carrot-pineapple juice on dental caries risk in children. Int. J. Probiotics Prebiotics. 2010;5:221–228. [Google Scholar]
- 202.Zahradnik RT, et al. Preliminary assessment of safety and effectiveness in humans of ProBiora3 TM, a probiotic mouthwash. J. Appl. Microbiol. 2009;107:682–690. doi: 10.1111/j.1365-2672.2009.04243.x. [DOI] [PubMed] [Google Scholar]