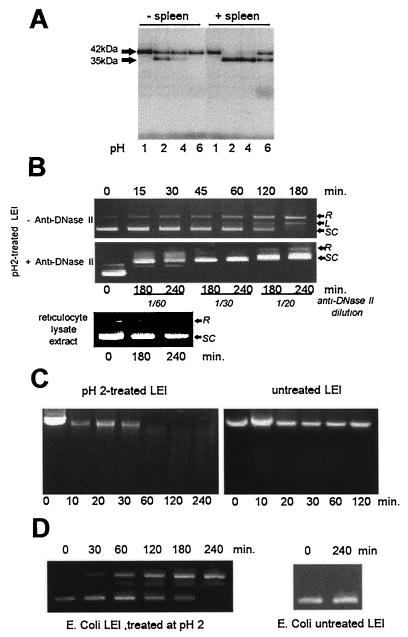
FIG. 4.
Posttranslation modification of porcine LEI. (A) Porcine LEI was expressed and [35S]methionine labeled with the TNT Promega reticulocyte lysate system. Aliquots of 3 μl were treated at 37°C overnight at different pHs in the presence or absence of crude porcine spleen extract. The reaction was stopped by adding the same volume of 2× Laemmli sample buffer. The samples were then separated on a 12% acrylamide gel and subjected to autoradiography. (B) A 4.5-μg sample of a supercoiled plasmid (pGEM) was incubated in 10 mM Tris–10 mM EDTA (pH 5.5) with 5 μl of the acid-treated protein; aliquots were ethanol precipitated at different incubation times, resuspended, and loaded on a 1% agarose gel (SC, supercoiled; L, linear; R, relaxed). The same experiment was performed, in the presence of anti-DNase II or with the TNT reticulocyte lysate alone, after treatment at pH 2.0. (C) DNase activity of pH 2-treated or untreated LEI was measured as in panel B, with genomic DNA as the substrate. (D) DNase activity was tested as in panel B, with a pH 2-treated LEI produced by E. coli and purified by the His-Bind system (Novagen).