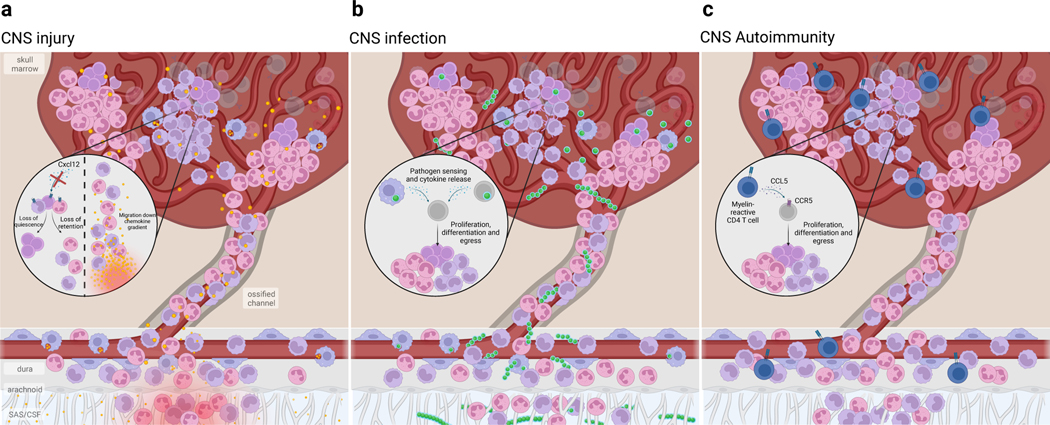
Fig. 3. Skull bone marrow responds to CNS perturbations.
Due to its proximity to the underlying CNS, the skull bone marrow can sense CNS impairment and mobilize immune supply to the underlying tissue. Alternatively, bone marrow niche perturbations can alter hematopoietic output to the CNS. a) Following CNS injury, such as spinal cord injury or stroke, HSPCs rapidly proliferate. This expands the monocyte and neutrophil supply into the underlying dura and injured CNS parenchyma. Such rapid response is due, in part, to CSF-contained cues entering the bone marrow and is likely a response to damage-associated molecular patterns (DAMP) resulting from tissue injury and cell death. Additionally, local retention cues such as Cxcl12 are downregulated, resulting in the mobilization of HSPCs and egress of monocytes and neutrophils. b) In bacterial meningitis, pathogens exploit the skull channel anatomy to invade the bone marrow. The rapid expansion of HSPCs as well as monocytes and neutrophils results from innate pathogen sensing, as well as local production of pro-inflammatory cytokines. c) In CNS autoimmune disease, autoreactive T cells home to the bone marrow and signal to hematopoietic stem cells to promote preferential over-production of monocytes and neutrophils. While this T cell homing and skewing of myelopoiesis seems to occur in both skull/vertebral bone marrow as well as femoral bone marrow, recent evidence suggests that skull/verterbal bone marrow-derived myeloid cells may be functionally distinct from their circulating counterparts during EAE. While there appears to be a conserved response in favor of myelopoiesis, whether these myeloid cells acquire phenotypes that are context-specific remains to be seen.