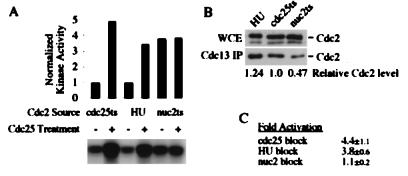
FIG. 3.
Cdc2 isolated from HU-arrested cells can be activated in vitro with Cdc25 phosphatase. (A) Cdc2-Cdc13 complexes were immunoprecipitated from HU-arrested cells (PR109) or from cells arrested by temperature sensitivity mutations in either cdc25-22 (GL84) or nuc2-226 (KS2020). These complexes were treated with human Cdc25C or mock treated and then were assayed for kinase activity on histone H1. The quantitation is normalized to the amount of Cdc2 present, as shown in panel B. (B) The amounts of Cdc2 in the anti-Cdc13 immunoprecipitates (IP) used in panel A, and the whole-cell extracts (WCE) from which they were derived, were quantitated by Western blotting with monoclonal antibodies against the Cdc2 PSTAIR epitope. The lower band in the whole-cell extract is a cross-reacting species that is not coprecipitated with Cdc13. (C) The fold activation represents the average and standard deviation from three independent experiments.