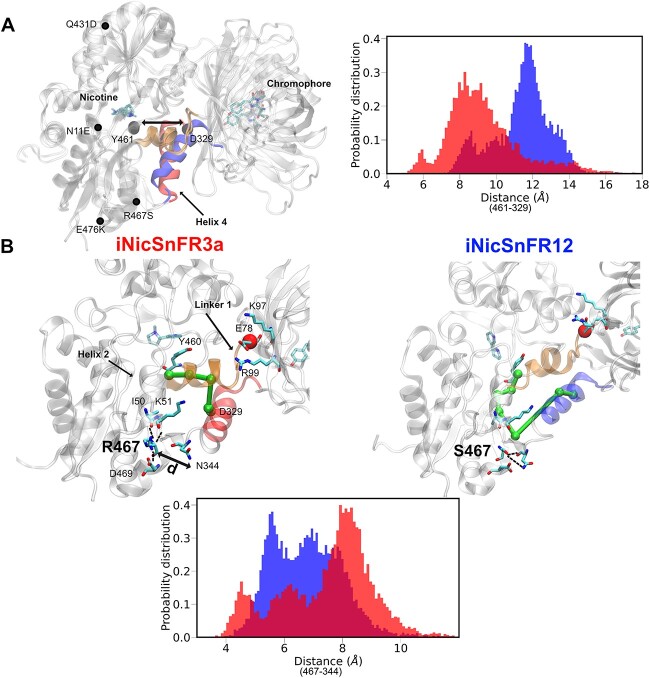
Fig. 4.
Tilting of linker helix 4 in iNicSnFR12 might modulate ligand binding. (A) All the mutations present in iNicSnFR12 from iNicSnFR3a are labeled. In iNicSnFR12, helix 4 (colored in blue) shifts toward the GFP. To measure this process, we analyzed the probability distribution of the distance between the Cα atom of a terminal residue D329 and the Cα atom Y461, the terminal of a β-sheet that locates nearest to helix 4, residue Y461. (B) The allosteric network from the nicotine binding site residue, Y460, to the N-terminal residue of helix 4, D329, is depicted in green. The probability distribution shows hydrogen bond interactions of residue 467 and N344, measured by calculating the distance between the side chains of these two residues in the two systems.