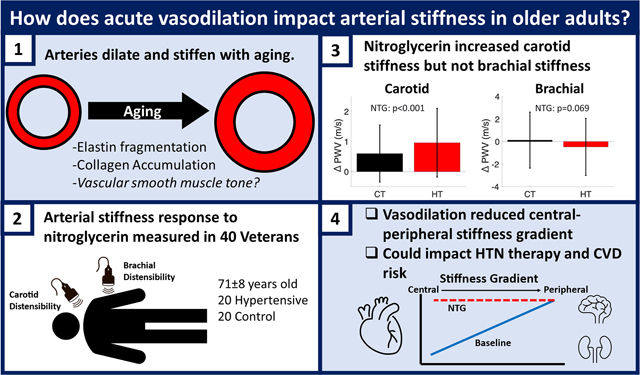
Abstract
Vascular smooth muscle tone may play an important role in the physiology of increased arterial stiffness that occurs with aging. This study evaluated the impact of smooth muscle tone on arterial stiffness in older individuals following nitroglycerin induced vasodilation in elastic and muscular arteries. 40 older Veterans (≥60 years old), without known cardiovascular disease, were included in this study. 20 were hypertensive (70.8±6.6 years, 10 female) and 20 were normotensive controls (72.0±9.3 years, 8 female). Nitroglycerin (NTG) induced changes in arterial stiffness were measured locally with vascular ultrasound in the carotid and brachial arteries, and regionally by carotid-femoral pulse wave velocity (cfPWV) by tonometry. With NTG, both hypertensive and normotensive control Veterans showed increased carotid PWV (6.4±1.3m/s to 7.2±1.4m/s, Δ 0.8±1.1m/s, p=0.007) and cfPWV (8.6±1.9m/s to 9.5±2.4m/s, Δ 0.9±2.3 m/s, p=0.020) but did not change brachial PWV (11.2±2.4m/s to 11.1±2.2m/s, Δ −0.2±2.5m/s, p=0.72). The carotid artery dilated more in control participants than hypertensive Veterans (Δ 0.54±0.19mm vs 0.42±0.12mm, p=0.022). Brachial artery dilation was similar, (Δ 0.55±0.26mm vs 0.51±0.20mm, p=0.46). In older Veterans, without known cardiovascular disease, NTG induced vasodilation increased elastic artery stiffness and did not change muscular artery stiffness. Increased central arterial stiffness and reduction in the arterial stiffness gradient could offset some of the benefits of lowering blood pressure in older patients who are prescribed vasodilators as an antihypertensive therapy. Elastic artery stiffening with vasodilation warrants further investigation as it may be important for antihypertensive medication selection and influence CVD development.
Keywords: Vascular stiffness, hypertension, vasodilation, smooth muscle, extracellular matrix
Graphical Abstract
Introduction
Studies of the biomechanics of the central elastic arteries like the aorta and carotid artery have predominately focused on passive contributions to arterial stiffness such as the extracellular matrix (ECM) and neglected active contributions to arterial stiffness from vascular smooth muscle (VSM)1. VSM is known to contribute significantly to the mechanical behavior of peripheral, muscular arteries. It has traditionally been believed that VSM and changes in VSM tone are unimportant to central, elastic artery stiffness1,2 due to their higher concentration of elastin3,4 and unique functional role to provide compliance and the Windkessel effect5,6. However, there is growing evidence that vascular smooth muscle has an important role in elastic artery mechanics. Animal studies show that vasoactive drugs can induce contractile responses in the aorta7 and the carotid artery7,8. While data in humans is more limited, and confounded by blood pressure, both nitroglycerin administration and lower limb venous occlusion in hypertensive adults have been found to invert the expected relationship of decreasing blood pressure leading to an decrease in arterial stiffness9,10. Together, these results indicate that VSM contraction may play a more important role in elastic artery function than traditionally thought.
In a recent retrospective analysis of several clinical studies, nitroglycerin (NTG) induced vasodilation was found to both increase and decrease arterial stiffness11 depending on the ratio of passive-to-active stiffness along the continuum of young-to-old, healthy-to-diseased, and elastic-to-muscular arteries studied. Giudici and Spronck proposed that the results of this retrospective analysis represent an important step towards understanding the role of VSM in large artery mechanics and function12. In particular, decreased VSM tone in the carotid artery of hypertensive individuals bore striking resemblance to the effects of 10 years of aging13,14, the carotid artery dilated and stiffened. These results suggest that VSM tone may be a key component of arterial aging and have different impacts on elastic versus muscular arteries. We performed the present study to test the responses of vasodilation and changes in VSM tone on arterial stiffness in the brachial and carotid arteries in older Veterans. NTG induced vasodilation was used because NTG is both quick acting and can safely be administered. We hypothesized that NTG induced vasodilation would increase elastic artery stiffness and decrease muscular artery stiffness.
Methods and Materials
This study was reviewed and approved by the University of Wisconsin and Madison Veterans Hospital Institutional Review Board. All participants gave written, informed consent to participate in this study.
Study Participants
This analysis included 40 Veteran participants (20 normotensive controls and 20 with a diagnosis of hypertension) from the ongoing Functional Arterial STiffness in Veterans (FAST-Vets) study at the Madison Veterans Affairs Hospital (MVAH). Ambulatory, community-dwelling, hypertensive, older Veterans (>60 years old) were recruited from the MVAH and surrounding clinics. Inclusion criteria were: male and female participants over the age 60 years from VA VISN 12, willing to undergo exercise treadmill stress testing, administration of sublingual NTG, 24-hour blood pressure monitoring, have tonometric evaluation of arterial stiffness, and undergo a brachial and carotid artery ultrasound as well as a non-invasive assessment of blood pressure and Cardiac Output (CO) using the Cheetah Starling System (Cheetah Medical, Inc. Newton Center, MA). Exclusion criteria were: known cardiovascular disease, secondary hypertension, chronic kidney disease, changing antihypertensive medication in the last month, active cancer (other than untreated, non-metastatic prostate cancer and non-melanoma skin cancer), hypoxemic pulmonary disease, active rheumatologic diseases (i.e., systemic lupus erythematosus, rheumatoid arthritis, etc.), human immunodeficiency virus, or illness with any infectious etiology or fever >38°C or hospitalization for any reason within the prior 4 weeks. Participants who were unable to walk on a treadmill were also excluded. For safety purposes, participants with baseline resting systolic BP < 110mmHg and mean arterial pressure <90mmHg or heart rate > 90 beats per minute were not administered nitroglycerin and were excluded from this analysis. A diagnosis of hypertension was assessed based on two or more office systolic BP measurements >140 mmHg, a mean home BP reading of >135 mmHg for over 7 days, or active use of antihypertensive medications.
Study Protocol
All studies were performed at the University of Wisconsin Atherosclerosis Imaging Research Program, which is a nationally recognized core ultrasound reading, training, and scanning laboratory. Visits began between 7AM and 11AM and participants withheld medications until after the study visit. For eight hours prior to the visit participants were asked to fast and refrain from smoking, caffeine, and sildenafil. On visit day 1 participants completed a maximal exercise treadmill stress test (data not shown). In between visit days 1 and 2 participants were fitted with a 24 hour ambulatory blood pressure monitor (ABPM) (SpaceLabs Healthcare, Snoqualmie, WA, USA) and were asked to wear the ABPM until they returned for their study visit the following day. 24 hour ABP was calculated as the mean of all the BP readings during both day and night.
On visit day 2 participants rested in a supine position in a temperature-controlled room for 10 minutes and serial baseline oscillometric brachial blood pressure (Cheetah Starling Fluid Management System, Baxter Healthcare, Deerfield, IL, USA) and radial artery tonometry (Atcor Sphygmacor, Atcor Medical, Sydney, Australia) were performed. Central systolic blood pressure was calculated from radial tonometry using a validated transfer function. Brachial artery flow mediated dilation (FMD) was performed following a forearm occlusion protocol (5 minutes occlusion period at 250mmHg) obtaining images of the right brachial artery 60 and 90 seconds post cuff release. FMD was calculated as the percent change from baseline brachial diameter to the maximum post cuff release diameter. Following a 20-minute wash out period post flow mediated dilation, baseline arterial stiffness measurements were performed in duplicate. Participants were then administered 400 micrograms sublingual NTG. Arterial stiffness measurements were repeated every 1-minute following NTG administration for at least 10 minutes or until the participant’s heart rate and blood pressure had returned to baseline (Figure 1).
Figure 1:
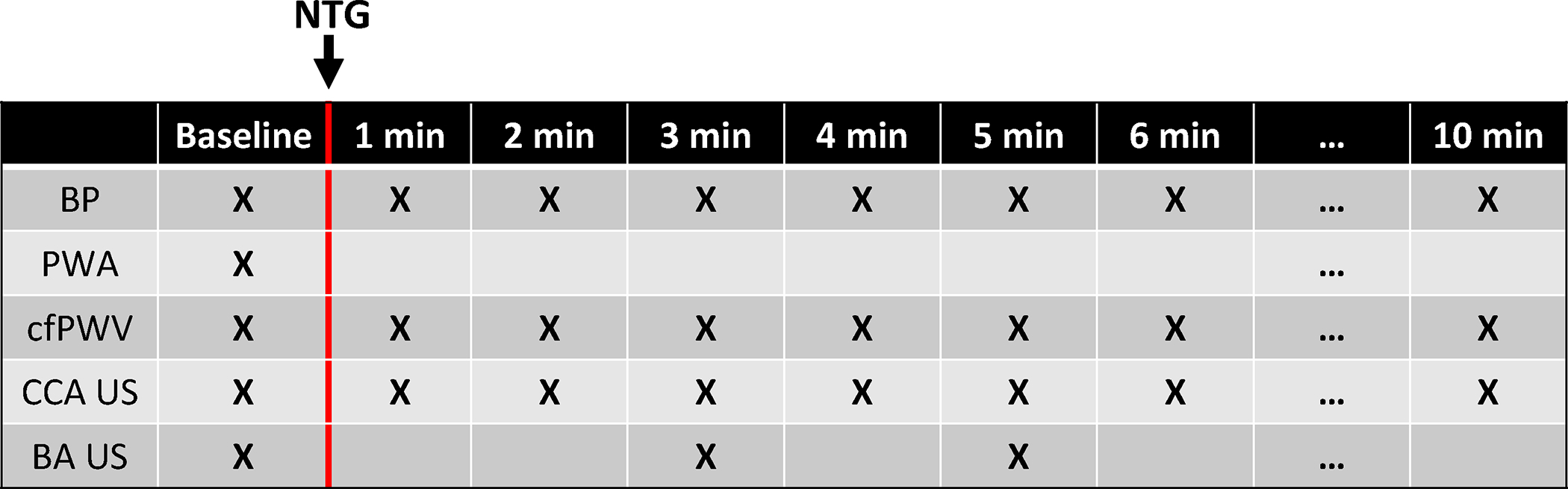
Diagram of the timeline for baseline and post-administration of 400 mcg sublingual nitroglycerin (NTG). After NTG administration, participants were monitored for at least 10 minutes or until blood pressure and heart rate returned to baseline. BP – Blood pressure, PWA – pulse wave analysis, cfPWV – carotid-femoral pulse wave velocity, CCA US – common carotid artery ultrasound, BA US – brachial artery ultrasound.
Arterial Stiffness Measurements
Carotid-to-femoral pulse wave velocity (cfPWV) was measured using an AtCor SphymoCor Px tonometry system (Atcor Medical, Sydney, Australia) which has a Millar micromanometer (Millar, SPT-301B, Houston, TX, USA). Transit distance to calculate cfPWV was measured as the carotid- suprasternal notch (SSN) distance subtracted from the SSN– femoral distance as recommended by AHA’s arterial stiffness scientific statement15. Vascular ultrasound was performed with a Philips CX-50 (Philips Ultrasound, Bothell, WA) ultrasound system with a 14L5 transducer by trained and registered sonographers from the University of Wisconsin Atherosclerosis Imaging Research Program. The brachial artery of the right arm was imaged 2–7 cm above the elbow and scanned in longitudinal sections with the focus set to the depth of the near wall. The brachial artery was imaged at baseline, 3 minutes post NTG, and 5 minutes post NTG. Longitudinal views of the distal 1cm of the right common carotid artery (CCA) were obtained with the imaging depth set at 4 cm, placing the artery between 2–3 cm deep on the screen, adjusting the focus and gain preferably stacking the jugular vein over the CCA to improve near and far wall resolution. B-mode ultrasound 5 beat DICOM clips were analyzed using Medical Imaging Applications Tools Carotid Analyzer (MIA Inc, Coralville, IA) software to measure far wall intima-media thickness (IMT) and artery lumen diameters over the cardiac cycles.
All baseline arterial stiffness measurements were performed in duplicate and averaged. The post NTG carotid and brachial artery stiffness measures were calculated from the two time points with the largest diastolic diameters which represent the time point with the maximal vasodilatory effect on the carotid or brachial artery. The post NTG carotid-femoral stiffness was calculated from the two time points with the greatest change in cfPWV from baseline. Blood pressure and heart rate were recorded from the measurement time point immediately prior to performing each arterial stiffness measurement. Because arterial stiffness measurements were performed at slightly different times the blood pressures and heart rates are also slightly different.
Arterial Stiffness Calculation and Modeling
Peterson’s Elastic Modulus () of the carotid and brachial arteries were calculated16:
| #(1) |
where represents the internal arterial diameter at peak systole, represents the internal diameter at end-diastole, and represents the brachial blood pressure difference between the systolic and diastolic measurements (pulse pressure). Local carotid and brachial pulse wave velocity (c/b PWV) was calculated from the Bramwell-Hill equation
| #(2) |
where is the density of blood (1050 kg/m3). Young’s elastic modulus () was then calculated from the Moens-Korteweg equation
| #(3) |
where IMT is the far wall artery wall intima-media thickness.
To control for changes in blood pressure with NTG administration, a participant-specific exponential model was used to describe the blood pressure dependence of arterial mechanics14,17. The mathematical equations are included as a supplement. Using the non-linear model, carotid artery and brachial artery stiffness metrics were calculated at a common 120/80 mmHg reference pressure pre- and post-NTG administration (Figure 2). The blood pressure dependence of cfPWV measurements were similarly adjusted using an exponential model. Guideline statements recommend adjusting cfPWV to MAP15 but because the waveform features used to calculate cfPWV occur at DBP it may be more appropriate to adjust cfPWV to DBP. We adjusted to both a DBP of 80mmHg and a MAP of 100mmHg.
Figure 2:
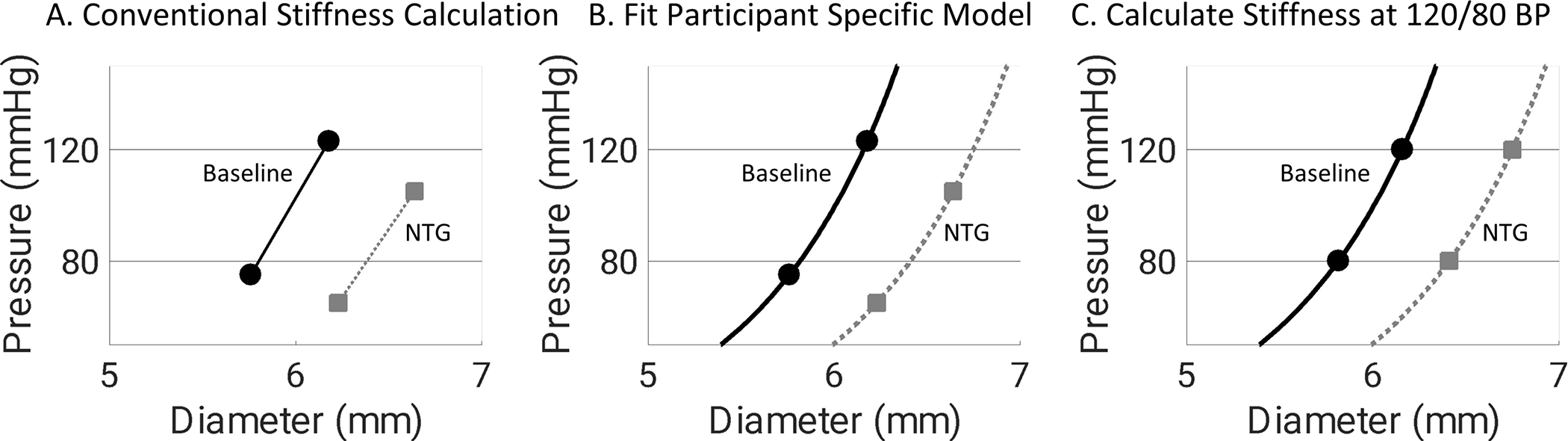
Graphical representation of the methods used to control for the blood pressure dependence of carotid artery stiffness for a representative participant. Baseline data are shown as black circles and NTG data is shown as gray squares. For conventional stiffness calculations NTG decreased Peterson’s elastic modulus (PEM) (318 to 296 mmHg) but blood pressure also decreased. NTG increased PEM (329 to 369 mmHg) after adjusting stiffness calculation at a constant 120/80mmHg blood pressure.
Lastly, we used a recently developed arterial mechanics model based on pressure-diameter relationships that incorporates the contributions to arterial stiffness from both the passive ECM and active VSM components of the arterial wall11. This model is advantageous because it’s simple enough to use with limited data available in clinical studies but has physiologically relevant parameters which include passive stiffness, active stiffness and active smooth muscle tone. The model was used to analyze the effects of nitroglycerin-induced vasodilation on carotid and brachial artery mechanics (Supplemental material).
Statistical Analysis
Continuous variables are presented as mean and standard deviation. Categorical variables are presented as number and percentage. Baseline characteristics for controls and hypertensive participants were compared using independent sample t-tests for continuous variables and χ2 tests for categorical variables. The arterial stiffness response to NTG was analyzed with a repeated-measures linear model with NTG administration as a within subjects factor and hypertension status as a between subjects factor. Additional repeated measured models were performed for sex, age, smoking status, and diabetes mellitus status). Results from modeling the active and passive contributions to arterial mechanics were compared using the Mann-Whitney U test due to non-normal distributions of some variables.
Results
Participant Characteristics
Participant characteristics are shown in Table 1. Controls and hypertensive Veterans participating in this study were similarly matched for age (72.0±9.3 vs 70.8±6.6 year, p=0.64), sex (40% female vs 50% female, p=0.53), and smoking status. 85% of hypertensive participants were using antihypertensive medications and self-reported an average of 14.7±14.3 years since hypertension diagnosis (range: 2–60 years). From 24-hour ambulatory blood pressure monitoring (ABPM), both SBP and DBP were not significantly different between hypertensive and control participants. 53% of control and 50% of hypertensive participants had well controlled 24-hour ABP (<125/75) according to 2017 AHA/ACC guidelines.
Table 1:
Participant Characteristics
| Control (n=20) | Hypertensive (n=20) | p-value | |
|---|---|---|---|
| Age (years) | 72.0±9.3 | 70.8±6.6 | 0.64 |
| BMI (kg/m2) | 27.6±4.5 | 27.0±6.5 | 0.72 |
| Sex | 0.53 | ||
| Female (n, %) | 8 (40%) | 10 (50%) | |
| Race/Ethnicity | 1.0 | ||
| White (n, %) | 19 (95%) | 19 (95%) | |
| Black (n, %) | 1 (5%) | 1 (5%) | |
| Diabetes Mellitus (n, %) | 2 (10%) | 6 (30%) | 0.11 |
| Smoking Status | 0.41 | ||
| Current (n, %) | 2 (10%) | 3 (15%) | |
| Former (n, %) | 9 (45%) | 12 (60%) | |
| Never (n, %) | 9 (45%) | 5 (25%) | |
| Pack Years | 14.7±19.2 | 16.7±18.7 | 0.74 |
| Supine Blood Pressure | |||
| SBP (mmHg) | 127±13 | 134±13 | 0.095 |
| DBP (mmHg) | 76±8 | 80±7 | 0.084 |
| MAP (mmHg) | 93±8 | 98±8 | 0.054 |
| cSBP (mmHg) | 119±10 (n=19) | 121±14 | 0.77 |
| 24hr ABPM | N=19 | N=16 | |
| 24hr SBP (mmHg) | 121±9 | 125±11 | 0.19 |
| 24hr DBP (mmHg) | 69±6 | 73±7 | 0.09 |
| 24hr BP < 125/75 (n, %) | 10 (53%) | 8 (50%) | 0.88 |
| Antihypertensive Medications (n,%) | -- | ||
| 0 | 3 (15%) | ||
| 1 | -- | 11 (55%) | |
| 2 | -- | 6 (30%) | |
| Antihypertensive Medication Classes (n,%) | -- | ||
| ACE Inhibitor | -- | 2 (10%) | |
| Angiotensin II Receptor Blocker | -- | 8 (40%) | |
| Calcium Channel Blocker | -- | 5 (25%) | |
| Beta Blocker | -- | 3 (15%) | |
| Thiazide Diuretic | -- | 3 (15%) | |
| Unknown | -- | 2 (10%) | |
| Self-Reported Years Since HT Diagnosis | -- | 14.7±14.3 | -- |
Abbreviations: BMI – body mass index, SBP – systolic blood pressure, DBP – diastolic blood pressure, MAP – mean arterial pressure, cSBP – central systolic blood pressure, ABPM – ambulatory blood pressure monitor, HT - Hypertension
Brachial Artery Stiffness
Brachial artery flow mediated dilation was not significantly different between control and hypertensive groups (4.14±2.48% vs 2.66±2.16%, p=0.054). For 6 control participants brachial artery ultrasound images were of insufficient quality to perform stiffness measurements. Brachial artery stiffness results are shown in Table 2, Figure 3, and individual participant data are presented in the data supplement (Figure S3). For both control and hypertensive groups nitroglycerin administration significantly decreased brachial blood pressure, increased heart rate, increased brachial artery diameter, and decreased brachial artery IMT (Table 2). Brachial artery PEM was similar between control and hypertensive groups at baseline (139±58vs 144±56kPa) and following nitroglycerin administration (141±48vs 133±49kPa). Nitroglycerin administration did not have a significant effect on brachial artery stiffness assessed by PEM, bPWV, and YEM (Table 2). After adjusting brachial artery stiffness parameters to a standard 120/80mmHg blood pressure, nitroglycerin administration significantly increased YEM120/80 (p=0.016) and had no effect on PEM120/80, or bPWV120/80. For brachial artery stiffness parameters there were no significant group differences or nitroglycerin-group interactions. No statistically significant effects were found for sex, age, smoking status, or diabetes mellitus status (Table S1–S4).
Table 2:
Brachial Artery Stiffness Response to Nitroglycerin
| Control (n=14) | Hypertensive (n=20) | p-value | |||||
|---|---|---|---|---|---|---|---|
| Baseline | NTG | Baseline | NTG | NTG | HT | NTG*HT | |
| SBP (mmHg) | 128±15 | 117±10 | 132±14 | 116±12 | <0.001 | 0.82 | 0.47 |
| DBP (mmHg) | 74±11 | 71±6 | 79±9 | 76±7 | 0.012 | 0.071 | 0.86 |
| MAP (mmHg) | 92±10 | 86±6 | 97±10 | 89±10 | <0.001 | 0.19 | 0.82 |
| HR (BPM) | 57±6 | 60±8 | 64±9 | 68±9 | <0.001 | 0.008 | 0.73 |
| DDia (mm) | 3.68±0.74 | 4.33±0.66 | 3.64±0.69 | 4.16±0.72 | <0.001 | 0.55 | 0.46 |
| IMT (mm) | 0.48±0.08 | 0.46±0.07 | 0.50±0.08 | 0.47±0.10 | 0.013 | 0.75 | 0.94 |
| PEM (kPa) | 139±58 | 141±48 | 144±56 | 133±49 | 0.69 | 0.93 | 0.56 |
| bPWV (m/s) | 11.2±2.4 | 11.4±2.1 | 11.5±2.2 | 11.0±2.2 | 0.72 | 0.93 | 0.47 |
| YEM (kPa) | 1075.9±630 | 1376±620 | 1116±561 | 1330±771 | 0.068 | 0.83 | 0.81 |
| PEM120/80 (kPa) | 142±70 | 153±53 | 137±53 | 140±50 | 0.57 | 0.56 | 0.71 |
| bPWV120/80 (m/s) | 11.3±2.7 | 11.9±2.2 | 11.2±2.0 | 11.3±2.2 | 0.48 | 0.62 | 0.55 |
| YEM120/80 (kPa) | 1122±699 | 1517±676 | 1072±559 | 1381±805 | <0.001 | 0.27 | 0.86 |
Abbreviations: NTG – nitroglycerin, HT – hypertension status, SBP – systolic blood pressure, DBP – diastolic blood pressure, MAP – mean arterial pressure, HR – heart rate, Ddia – diastolic diameter, IMT – intima-media thickness, PEM – Peterson’s elastic modulus, bPWV – brachial pulse wave velocity, YEM – Young’s elastic modulus, 120/80 subscript – adjusted to 120/80 blood pressure using participant specific model
Figure 3:
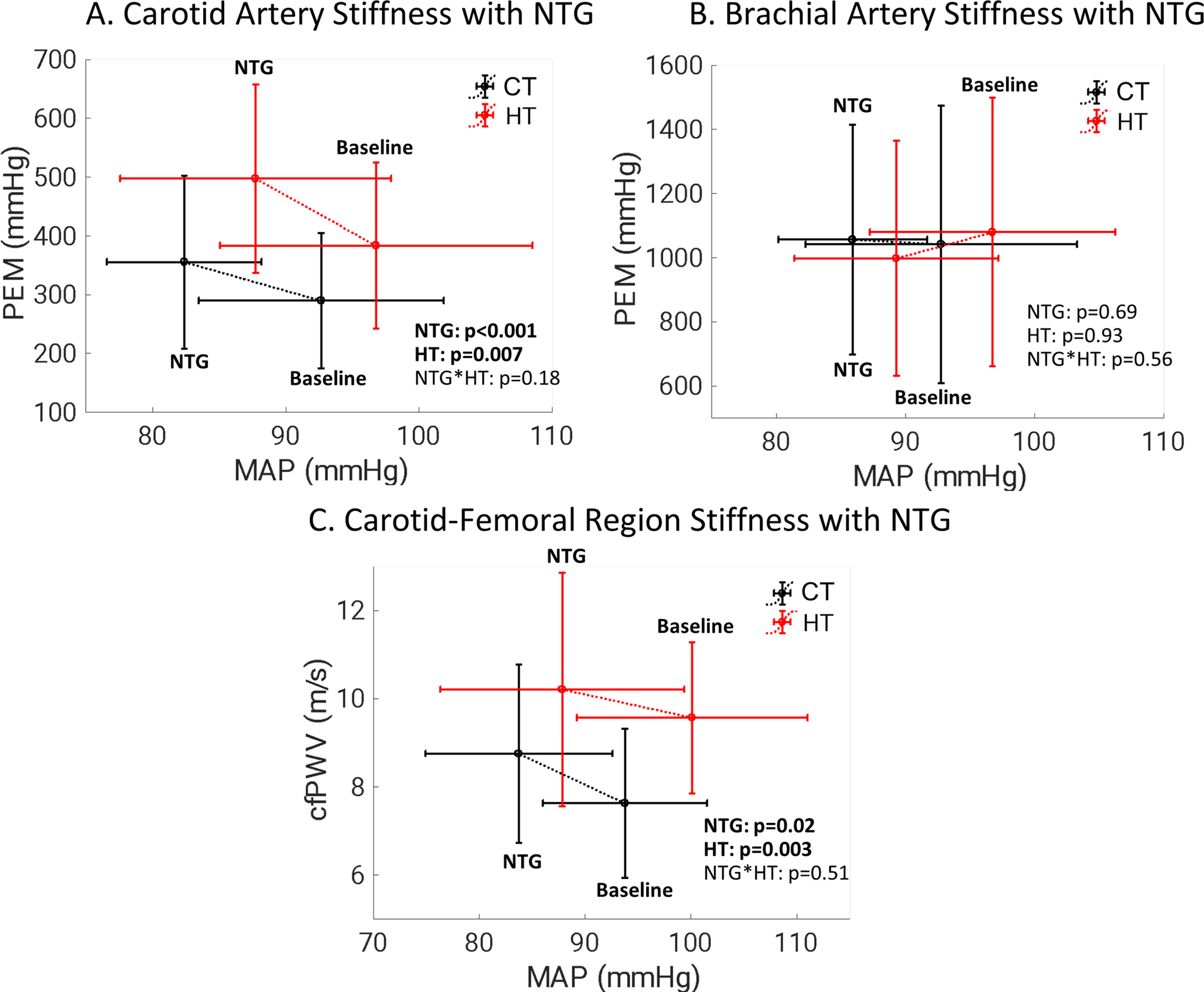
Despite causing approximately 10mmHg decreases in mean arterial pressure (MAP), nitroglycerin (NTG) administration A. increased carotid artery Petersons’ elastic modulus (PEM), B. did not change brachial artery PEM, and C. increased carotid-femoral pulse wave velocity (cfPWV). Abbreviations: CT – Control, HT – hypertensive
Carotid Artery Stiffness
Carotid artery stiffness results are shown in Table 3, Figure 3, and individual participant data are presented in the data supplement (Figure S2). For both control and hypertensive groups, nitroglycerin administration significantly decreased blood pressure, increased heart rate, increased carotid artery diameter, and decreased carotid artery IMT (Table 3). Carotid artery PEM was lower in control participants compared to hypertensive participants at baseline (38.5±15.3vs 51.1±18.8 kPa) and following nitroglycerin administration (47.3±19.6 vs 65.9±21.5 kPa). Nitroglycerin administration significantly increased carotid artery stiffness assessed by PEM, cPWV, and YEM before and after adjusting carotid artery stiffness parameters to a standard 120/80mmHg blood pressure (Table 3). Hypertensive participants had significantly elevated (p<0.05) PEM, cPWV, YEM, PEM120/80, and cPWV120/80 compared to control participants. No significant nitroglycerin-group interactions were observed for carotid artery stiffness parameters. The only statistically significant nitroglycerin-group interaction for the carotid artery was that control participants had a greater increase in diastolic diameter compared to hypertensive participants following nitroglycerin administration (Δ: 0.54±0.19mm vs 0.42±0.12mm, p=0.022). No statistically significant effects were found for sex, age, smoking status, or diabetes mellitus status (Table S1–S4).
Table 3:
Carotid Artery Stiffness Response to Nitroglycerin
| Control (n=20) | Hypertensive (n=20) | p-value | |||||
|---|---|---|---|---|---|---|---|
| Baseline | NTG | Baseline | NTG | NTG | HT | NTG*HT | |
| SBP (mmHg) | 128±14 | 110±12 | 134±17 | 117±16 | <0.001 | 0.11 | 0.81 |
| DBP (mmHg) | 75±9 | 71±6 | 78±11 | 76±7 | <0.001 | 0.066 | 0.51 |
| MAP (mmHg) | 93±9 | 82±6 | 97±12 | 88±10 | <0.001 | 0.090 | 0.65 |
| HR (BPM) | 58±8 | 60±8 | 64±9 | 68±9 | <0.001 | 0.022 | 0.92 |
| DDia (mm) | 5.80±0.73 | 6.34±0.76 | 6.16±0.68 | 6.58±0.68 | <0.001 | 0.19 | 0.022 |
| IMT (mm) | 0.73±0.12 | 0.70±0.14 | 0.83±0.16 | 0.78±0.15 | 0.002 | 0.051 | 0.341 |
| PEM (kPa) | 38.5±15.3 | 47.3±19.6 | 51.1±18.8 | 65.9±21.5 | <0.001 | 0.007 | 0.18 |
| cPWV (m/s) | 6.0±1.1 | 6.6±1.3 | 6.8±1.3 | 7.8±1.3 | <0.001 | 0.007 | 0.29 |
| YEM (kPa) | 312±139 | 439±182 | 378±127 | 565±180 | <0.001 | 0.036 | 0.21 |
| PEM120/80 (kPa) | 39.9±15.9 | 55.9±22.9 | 48.4±15.1 | 70.8±19.2 | <0.001 | 0.030 | 0.24 |
| cPWV120/80 (m/s) | 6.1±1.1 | 7.2±1.4 | 6.7±1.1 | 8.1±1.1 | <0.001 | 0.022 | 0.37 |
| YEM120/80 (kPa) | 328±144 | 533±196 | 365±110 | 617±172 | <0.001 | 0.16 | 0.39 |
Abbreviations: NTG – nitroglycerin, HT – hypertension status, SBP – systolic blood pressure, DBP – diastolic blood pressure, MAP – mean arterial pressure, HR – heart rate, Ddia – diastolic diameter, IMT – intima-media thickness, PEM – Peterson’s elastic modulus, cPWV – carotid pulse wave velocity, YEM – Young’s elastic modulus, 120/80 subscript – adjusted to 120/80 blood pressure using participant specific model
Carotid-Femoral Region Stiffness
Carotid-femoral region stiffness results are shown in Table 4, Figure 3, and individual participant data are presented in the data supplement (Figure S4). For both the control and hypertensive participants nitroglycerin administration significantly decreased SBP (Δ: −20±14 mmHg, p<0.001), DBP (Δ: −7±8 mmHg, p<0.001), and MAP (Δ: −11±9 mmHg, p<0.001) (Table 4. cfPWV was significantly lower in control participants compared to hypertensive participants at baseline (7.6±1.7 vs 9.6±1.8 m/s) and following nitroglycerin administration (8.7±2.0 vs 10.2±2.6 m/s). Nitroglyerin administration significantly increased cfPWV in both groups (Δ: 0.9±2.3 m/s, p=0.020). Hypertensive participants had significantly elevated (p<0.05) cfPWV, cfPWV80, and cfPWV100 compared to control participants. No significant nitroglycerin-group interactions were observed for carotid-femoral region stiffness parameters. No statistically significant effects were found for sex, age, smoking status, or diabetes mellitus status (Table S1–S4).
Table 4:
Carotid-Femoral Region Stiffness Response to Nitroglycerin
| Control (n=20) | Hypertensive (n=20) | p-value | |||||
|---|---|---|---|---|---|---|---|
| Baseline | NTG | Baseline | NTG | NTG | HT | NTG*HT | |
| SBP (mmHg) | 129±10 | 110±16 | 138±18 | 118±17 | <0.001 | 0.067 | 0.84 |
| DBP (mmHg) | 76±9 | 69±7 | 81±8 | 73±10 | <0.001 | 0.065 | 0.48 |
| MAP (mmHg) | 94±8 | 84±9 | 100±11 | 88±12 | <0.001 | 0.081 | 0.54 |
| cfPWV (m/s) | 7.6±1.7 | 8.7±2.0 | 9.6±1.8 | 10.2±2.6 | 0.02 | 0.003 | 0.51 |
| cfPWV80 (m/s) | 7.9±1.7 | 9.9±1.8 | 9.5±1.8 | 11.4±2.4 | <0.001 | 0.005 | 0.74 |
| cfPWV100 (m/s) | 7.9±1.7 | 10.3±1.9 | 9.6±1.7 | 11.6±2.4 | <0.001 | 0.007 | 0.68 |
Abbreviations: NTG – nitroglycerin, HT – hypertension status, SBP – systolic blood pressure, DBP – diastolic blood pressure, MAP – mean arterial pressure, cfPWV, 80 subscript – adjusted to 80mmHg diastolic blood pressure using participant specific model, 100 subscript – adjusted to 100mmHg mean arterial pressure using participant specific model
Passive versus Active Arterial Mechanics
A two-term exponential model was applied to nitroglycerin vasodilation data from each participant to determine the passive ECM and active VSM contributions to arterial mechanics of the carotid and brachial arteries. Carotid artery data for one control participant and two hypertensive participant were excluded from analysis of active stiffness and basal smooth muscle tone due to residual error after parameter fitting (Supplemental Material). Modeling results are shown in Figure 4. For the carotid artery, hypertensive participants had greater passive stiffness compared to control participants (10.8±2.7 vs 8.5±3.4, p=0.003, dimensionless) while active stiffness was not significantly different (5.7±3.6 vs 4.5±2.8, p=0.44, dimensionless). For the brachial artery, hypertensive and control participants had similar passive and active stiffness (Figure 4). Carotid artery basal smooth muscle tone (parameter k in model) was lower in hypertensive participants (0.052± 0.013 vs 0.062 ± 0.017, p=0.019, dimensionless). Brachial artery basal smooth muscle tone was similar between groups (0.115± 0.038 vs 0.117 ± 0.052 p=0.96, dimensionless). In the brachial artery 86.7±14.1% of baseline SBP was supported by active elements of the artery wall compared to 43.9±13.0% in the carotid artery. For both the carotid artery and the brachial artery, the percentage of baseline SBP supported by active muscular elements of the artery wall was not significantly different in hypertensive participants compared to control participants (Figure 4).
Figure 4:
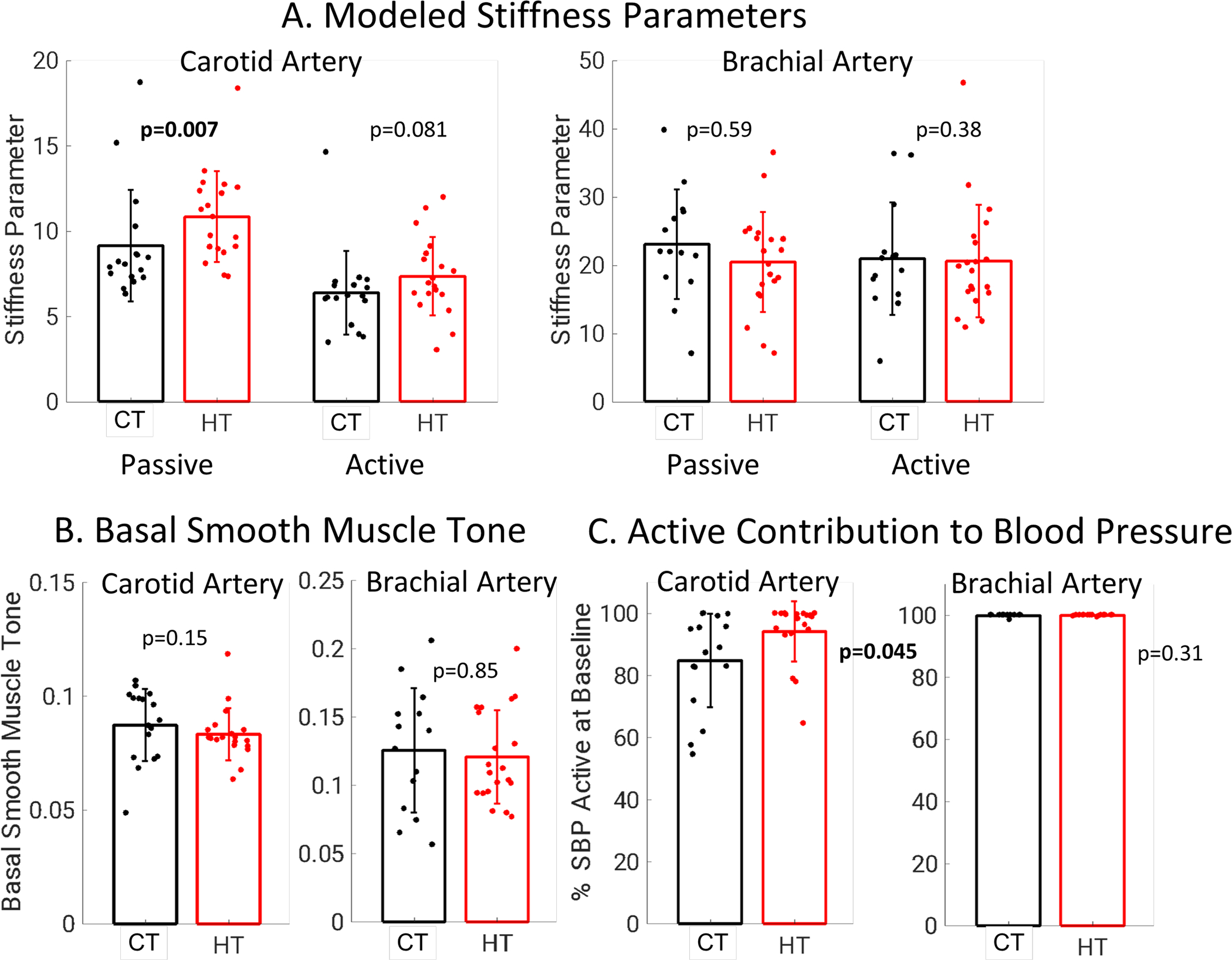
A. Carotid artery passive stiffness from modeling was elevated in hypertensive participants while carotid artery active stiffness, brachial artery passive stiffness, and brachial artery active stiffness were not different between groups. B. Carotid artery basal smooth muscle tone was lower in hypertensive participants and brachial artery basal smooth muscle tone was similar between groups. C. For the carotid artery, a similar percentage of baseline SBP was supported by active and passive elements of the artery wall. For the brachial artery, the majority of baseline SBP was supported by active components of the artery wall for both groups.
Discussion
This study assessed the effects of nitroglycerin induced vasodilation on the stiffness of elastic and muscular arteries in older Veterans. The major finding was that the stiffness of central, elastic arteries significantly increased following sublingual nitroglycerin administration. Based on prior studies examining overall CVD risk associated with arterial stiffness parameters, this is a clinically significant observation that equates to an approximate increase of 15% in CVD risk18. In contrast to the carotid artery and carotid-femoral region, nitroglycerin did not change the stiffness of the muscular brachial artery.
In this study vasodilator administration decreased brachial BP by approximately 10 mmHg, but also had the negative effect of paradoxically increasing elastic artery stiffness. This result was confirmed with two independent measures of elastic artery stiffness, carotid ultrasound and cfPWV. Vasodilator administration also did not change muscular artery stiffness, which would reduce the central-to-peripheral arterial stiffness gradient and increase pulsatile energy transmitted to the microvasculature19. Awareness of the increase in central arterial stiffness and reduction in the arterial stiffness gradient, despite a decrease in BP, is paramount because it could offset some of the benefits of lowering BP in older patients who are prescribed vasodilators as an antihypertensive therapy. These results emphasize that direct vasodilators should be reserved as 4th-5th line antihypertensive therapy options, particularly in older patients, and prompt the need to test the impact of other hypertensive therapies on active and passive stiffness parameters. Angiotensin converting enzyme inhibitors have a greater effect on decreasing arterial stiffness compared to calcium channel blockers and beta blockers20 which could be due to different effects on elastic artery smooth muscle tone.
Nitroglycerin induced vasodilation also had different effects on the stiffness of elastic versus muscular arteries, likely due to their different functions and the ratio of active stiffness of vascular smooth muscle cells versus passive stiffness of the ECM. Vasodilation increased carotid artery stiffness by 27% and did not change brachial artery stiffness. Our results in older Veterans show different effects of nitroglycerin on arterial stiffness than previous studies in younger to middle-aged adults. Nitroglycerin decreased elastic artery stiffness in younger control individuals9,21 and either did not change9 or increased elastic artery stiffness in younger hypertensive individuals22. In younger non-hypertensive individuals, nitroglycerin decreased muscular artery stiffness21,23,24. These results cannot solely be due to the straightening of collagen fibers with dilation as then vasodilation would be correlated with increased stiffness. We did not find changes in arterial diameter were correlated with changes in arterial stiffness (Supplemental Figure S5). This is in contrast to previous results showing that increased radial artery diameter with vasoactive drugs was correlated with decreased brachial-radial PWV in young men25. In addition to the differences in age, sex, and the arteries studied, statistical analysis is another potential reason for this difference. Fok et al., analyzed correlations of the average group response to different drugs and doses, which may be different from the correlations of the individual participant response.
We hypothesize that the different stiffness response of elastic versus muscular artery stiffness to vasodilation are due to the ratio of passive and active contributions to arterial stiffness based on a Hill-type partition of passive-elastic and active-contractile forces11 (Figure 5). When an artery has low passive stiffness compared to active stiffness, decreasing VSM tone will decrease arterial stiffness. In contrast, when an artery has high passive stiffness compared to active stiffness, decreasing VSM tone will increase arterial stiffness. Our results are in line with this hypothesis as the elastic arteries of older individuals likely have higher passive stiffness due to mechanisms such as collagen accumulation and elastin fragmentation26. The brachial artery transition from NTG decreasing stiffness in young men to NTG not changing stiffness in older Veterans would indicate that with aging the brachial artery remodels from having stiffer VSM than ECM to having relatively equal VSM and ECM stiffness.
Figure 5:
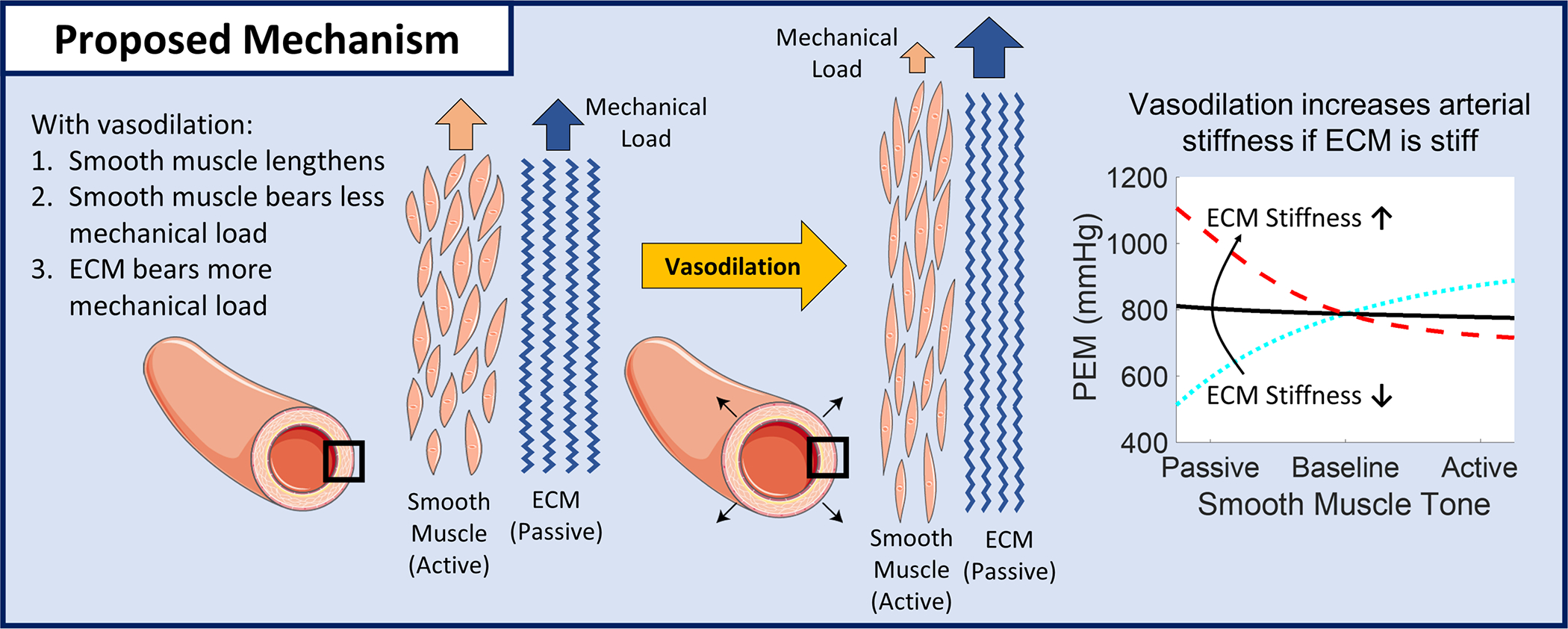
Proposed mechanism for elastic artery stiffening with vasodilation in older adults. With vasodilation, decreasing smooth muscle tone, vascular smooth muscle supports less mechanical load and the ECM supports more mechanical load. If the ECM is stiffer than the smooth muscle (as is likely the case in older adults), vasodilation shifting load from smooth muscle to the ECM will make the artery stiffer. Figure created with images from Servier Medical Art35.
The arterial stiffness response to vasodilation is likely important in the development of CVD. Among individuals suspected of coronary artery disease (CAD), those in whom NTG increased carotid artery stiffness had both greater prevalence and severity of angiographic coronary artery disease compared to those in whom NTG decreased carotid artery stiffness27. The older Veterans in this study were free from known CVD, so the abnormal response of vasodilation increasing carotid artery stiffness could be a precursor to CVD development and may represent a novel risk marker. Changes in elastic artery VSM tone could have adaptive or maladaptive effects on pathological arterial remodeling in hypertension. In our analyses, the carotid artery basal smooth muscle tone was lower in hypertensive participants. Increased VSM tone has been shown to reduce arterial stiffness and reduce excess stress on the ECM which promotes adaptive arterial remodeling in the carotid of hypertensive rats28 and the thoracic aorta remodeling in hypertensive mice29. In rodents, increased VSM tone in hypertension is an acute phenomenon, only lasting a handful of weeks. Our data shows the opposite effect (decreased VSM tone) in older Veterans who have had hypertension for years. Decreased elastic artery VSM tone in hypertensive individuals could also partially explain increased carotid artery diameter in hypertension. The average difference in carotid artery diameter versus controls was smaller with vasodilation (0.24 mm) compared to baseline (0.36mm). This suggests that basal VSM tone may be a therapeutic target to promote adaptive arterial remodeling in humans with hypertension.
Arterial stiffness is implicated in the pathway from isolated systolic hypertension to heart failure with preserved ejection fraction (HFpEF) in older individuals30 and development of isolated systolic hypertension through decreased arterial compliance31. Abnormal arterial stiffness responses to physiologic and pharmacologic changes can alter left ventricle afterload and exercise capacity and ultimately lead to left ventricular dysfunction32. It may be possible to beneficially alter these negative outcomes by modifying arterial stiffness through VSM tone.
Limitations of this study include the relatively small number of subjects and is limited to an older, albeit less studied, patient population. Peripheral brachial artery BP was used instead of central carotid artery BP to calculate carotid artery stiffness. Nitroglycerin can have a greater impact on central BP compared to peripheral BP33 although the difference between central and peripheral BP should be lower in older individuals. We did not correct for changes in heart rate, but the increase in heart rate was small (3.5±4.5 BPM). Based on past studies34, this increase in heart rate would only account for a cfPWV increase of approximately 0.05 m/s while we found cfPWV increased by 0.9 m/s. A washout period for antihypertensive medications prior to the study visit was not used. Even though participants did not take medications the morning of their visit, medications taken the prior day could exert some vasoactive effects and influence results. Finally, no histological data was collected or evaluated.
Perspectives
In older Veterans, without known cardiovascular disease, NTG induced vasodilation increased elastic artery stiffness and did not change muscular artery stiffness. Elastic artery stiffening with vasodilation may be important for antihypertensive medication selection and influence CVD development and should be a focus of future studies.
Supplementary Material
Acknowledgements:
We would like to thank the Veterans who volunteered to participate in this study and Kristin Hansen for performing vascular ultrasound exams.
Funding:
This work was supported by a Career Development Award IK2-CX001776 from the United States (U.S.) Department of Veterans Affairs Clinical Sciences Research and Development Service. This material is the result of work supported with resources and the use of facilities at the Madison VA Hospital and the University of Wisconsin Atherosclerosis Imaging Research Program. Dr. Pewowaruk was supported by a T32 HL 07936 Ruth L. Kirschstein National Research Service Award from the National Heart Lung and Blood Institute to the University of Wisconsin-Madison Cardiovascular Research Center. The contents do not represent the views of the U.S. Department of Veterans Affairs, NHLBI, or the United States Government.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest, financial or otherwise, to disclose.
References
- 1.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol. Rev. 2009; 89: 957–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lacolley P, Regnault V, Segers P, Laurent S. Vascular smooth muscle cells and arterial stiffening: Relevance in development, aging, and disease. Physiol Rev 2017; 97: 1555–1617. doi: 10.1152/physrev.00003.2017 [DOI] [PubMed] [Google Scholar]
- 3.HARKNESS ML HARKNESS RD, MCDONALD DA. The collagen and elastin content of the arterial wall in the dog. Proc R Soc Lond B Biol Sci 1957; 146: 541–551. doi: 10.1098/rspb.1957.0029 [DOI] [PubMed] [Google Scholar]
- 4.BURTON AC. Relation of structure to function of the tissues of the wall of blood vessels. Physiol Rev 1954; 34: 619–642. doi: 10.1152/physrev.1954.34.4.619 [DOI] [PubMed] [Google Scholar]
- 5.Sagawa K, Lie RK, Schaefer J. Translation of Otto frank’s paper ‘Die Grundform des arteriellen Pulses’ zeitschrift für biologie 37: 483–526 (1899). J Mol Cell Cardiol 1990; 22: 253–254. doi: 10.1016/0022-2828(90)91459-K [DOI] [PubMed] [Google Scholar]
- 6.Westerhof N, Stergiopulos N, Noble MIM. The Arterial Windkessel. In Snapshots of Hemodynamics: An Aid for Clinical Research and Graduate Education.2010, 173–181. [Google Scholar]
- 7.Leloup AJA, Van Hove CE, Heykers A, Schrijvers DM, De Meyer GRY, Fransen P. Elastic and muscular arteries differ in structure, basal NO production and voltage-gated Ca2+-channels. Front Physiol 2015; 6. doi: 10.3389/fphys.2015.00375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner HP, Humphrey JD. Differential passive and active biaxial mechanical behaviors of muscular and elastic arteries: Basilar versus common carotid. J Biomech Eng 2011; 133. doi: 10.1115/1.4003873 [DOI] [PubMed] [Google Scholar]
- 9.Stewart AD, Jiang B, Millasseau SC, Ritter JM, Chowienczyk PJ. Acute reduction of blood pressure by nitroglycerin does not normalize large artery stiffness in essential hypertension. Hypertension 2006; 48: 404–410. doi: 10.1161/01.HYP.0000237669.64066.c5 [DOI] [PubMed] [Google Scholar]
- 10.Faconti L, Farukh B, McNally R, Webb A, Chowienczyk P. Arterial Stiffness Can Be Modulated by Pressure-Independent Mechanisms in Hypertension. J Am Heart Assoc 2019; 8: e012601. doi: 10.1161/JAHA.119.012601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pewowaruk RJ, Gepner AD. Smooth muscle tone alters arterial stiffness. J Hypertens 2021; Publish Ah: 1–8. doi: 10.1097/hjh.0000000000003039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giudici A B S. A first step towards recognizing the fundamental role of smooth muscle tone in large artery (dys)function? J Hypertens 2022; 40: 422–424. doi: 10.1097/HJH.0000000000003063 [DOI] [PubMed] [Google Scholar]
- 13.Gepner AD, Korcarz CE, Colangelo LA, Hom EK, Tattersall MC, Astor BC, Kaufman JD, Liu K, Stein JH. Longitudinal effects of a decade of aging on carotid artery stiffness : The multiethnic study of atherosclerosis. Stroke 2014; 45: 48–53. doi: 10.1161/STROKEAHA.113.002649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pewowaruk RJ, Tedla Y, Korcarz CE, Tattersall MC, Stein JH, Chesler NC, Gepner AD. Carotid Artery Stiffening With Aging: Structural Versus Load-Dependent Mechanisms in MESA (the Multi-Ethnic Study of Atherosclerosis). Hypertension 2021; : 1–9. doi: 10.1161/hypertensionaha.121.18444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement from the American Heart Association. Hypertension 2015; 66: 698–722. doi: 10.1161/HYP.0000000000000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J 2006; 27: 2588–2605. doi: 10.1093/eurheartj/ehl254 [DOI] [PubMed] [Google Scholar]
- 17.Van Der Bruggen M, Spronck B, Bos S, Heusinkveld MHG, Taddei S, Ghiadoni L, Delhaas T, Bruno RM, Reesink KD. Pressure-Corrected Carotid Stiffness and Young’s Modulus: Evaluation in an Outpatient Clinic Setting. Am J Hypertens 2021; 34: 737–743. doi: 10.1093/ajh/hpab028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of Cardiovascular Events and All-Cause Mortality With Arterial Stiffness. A Systematic Review and Meta-Analysis. J Am Coll Cardiol 2010; 55: 1318–1327. doi: 10.1016/j.jacc.2009.10.061 [DOI] [PubMed] [Google Scholar]
- 19.Fortier C, Agharazii M. Arterial Stiffness Gradient. Pulse 2015; 3: 159–166. doi: 10.1159/000438852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong KT, Delerme S, Pannier B, Safar ME, Benetos A, Laurent S, Boutouyrie P. Aortic stiffness is reduced beyond blood pressure lowering by short-term and long-term antihypertensive treatment: A meta-analysis of individual data in 294 patients. J Hypertens 2011; 29: 1034–1042. doi: 10.1097/HJH.0b013e328346a583 [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto T, Shimizu K, Takahashi M, Tatsuno I, Shirai K. The effect of nitroglycerin on arterial stiffness of the aorta and the femoral-tibial arteries: -Monitoring with a stiffness parameter β- derived vascular index. J Atheroscler Thromb 2017; 24: 1048–1057. doi: 10.5551/jat.38646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soma J, Angelsen BAJ, Techn D, Aakhus S, Skjærpe T. Sublingual nitroglycerin delays arterial wave reflections despite increased aortic ‘stiffness’ in patients with hypertension: A Doppler Echocardiography study. J Am Soc Echocardiogr 2000; 13. doi: 10.1067/mje.2000.109686 [DOI] [PubMed] [Google Scholar]
- 23.Bank AJ, Wilson RF, Kubo SH, Holte JE, Dresing TJ, Wang H. Direct effects of smooth muscle relaxation and contraction on in vivo human brachial artery elastic properties. Circ Res 1995; 77: 1008–1016. doi: 10.1161/01.RES.77.5.1008 [DOI] [PubMed] [Google Scholar]
- 24.Kaiser DR, Mullen K, Bank AJ. Brachial artery elastic mechanics in patients with heart failure. Hypertension 2001; 38: 1440–1445. doi: 10.1161/hy1201.096539 [DOI] [PubMed] [Google Scholar]
- 25.Fok H, Jiang B, Clapp B, Chowienczyk P. Regulation of vascular tone and pulse wave velocity in human muscular conduit arteries: Selective effects of nitric oxide donors to dilate muscular arteries relative to resistance vessels. Hypertension 2012; 60. doi: 10.1161/HYPERTENSIONAHA.112.198788 [DOI] [PubMed] [Google Scholar]
- 26.O’Rourke MF, Hashimoto J. Mechanical Factors in Arterial Aging. A Clinical Perspective. J Am Coll Cardiol 2007; 50: 1–13. doi: 10.1016/j.jacc.2006.12.050 [DOI] [PubMed] [Google Scholar]
- 27.Lai CP, Koyanagi S, Shaw CK, Takeshita A. Evaluation of the early stage of carotid atherosclerosis using the vascular response to nitroglycerin and high-resolution ultrasonography. Jpn Circ J 1998; 62: 494–498. doi: 10.1253/jcj.62.494 [DOI] [PubMed] [Google Scholar]
- 28.Fridez P, Makino A, Kakoi D, Miyazaki H, Meister JJ, Hayashi K, Stergiopulos N. Adaptation of conduit artery vascular smooth muscle tone to induced hypertension. Ann Biomed Eng 2002; 30: 905–916. doi: 10.1114/1.1507326 [DOI] [PubMed] [Google Scholar]
- 29.Spronck B, Latorre M, Wang M, Mehta S, Caulk AW, Ren P, Ramachandra AB, Murtada S Il, Rojas A, He CS, Jiang B, Bersi MR, Tellides G, Humphrey JD. Excessive adventitial stress drives inflammation-mediated fibrosis in hypertensive aortic remodelling in mice. J R Soc Interface 2021; 18. doi: 10.1098/rsif.2021.0336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desai AS, Mitchell GF, Fang JC, Creager MA. Central Aortic Stiffness is Increased in Patients With Heart Failure and Preserved Ejection Fraction. J Card Fail 2009; 15. doi: 10.1016/j.cardfail.2009.03.006 [DOI] [PubMed] [Google Scholar]
- 31.Safar ME. Systolic hypertension in the elderly: Arterial wall mechanical properties and the renin-angiotensin-aldosterone system. J. Hypertens. 2005; 23: 673–681. [DOI] [PubMed] [Google Scholar]
- 32.Zern EK, Ho JE, Panah LG, Lau ES, Liu E, Farrell R, Sbarbaro JA, Schoenike MW, Pappagianopoulos PP, Namasivayam M, Malhotra R, Nayor M, Lewis GD. Exercise Intolerance in Heart Failure With Preserved Ejection Fraction: Arterial Stiffness and Aabnormal Left Ventricular Hemodynamic Responses During Exercise. J Card Fail 2021; 27. doi: 10.1016/j.cardfail.2021.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly RP, Gibbs HH, O’Rourke MF, Daley JE, Mang K, Morgan JJ, Avolio AP. Nitroglycerin has more favourable effects on left ventricular afterload than apparent from measurement of pressure in a peripheral artery. Eur Heart J 1990; 11: 138–144. doi: 10.1093/oxfordjournals.eurheartj.a059669 [DOI] [PubMed] [Google Scholar]
- 34.Tan I, Spronck B, Kiat H, Barin E, Reesink KD, Delhaas T, Avolio AP, Butlin M. Heart Rate Dependency of Large Artery Stiffness. Hypertension 2016; 68: 236–242. doi: 10.1161/HYPERTENSIONAHA.116.07462 [DOI] [PubMed] [Google Scholar]
- 35.Servier LL. Servier Medical Art. smart.servier.com
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


