Figure 1.
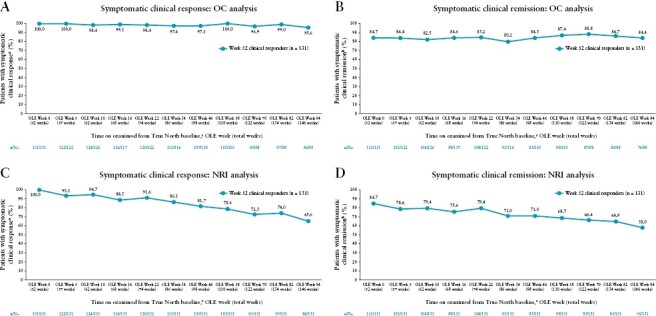
Proportions of patients on continuous ozanimod who entered the OLE in clinical response achieving symptomatic clinical response and symptomatic clinical remission over time in the OLE through OLE Week 94. [A, B] OC analysis. [C, D] NRI analysis. Denominators for the OC analyses were based on the numbers of patients who completed OLE Week 5, 10, 16, 22, 34, 46, 58, 70, 82, or 94 and had data available for the endpoints in question. Denominators for the NRI analyses were based on the numbers of patients who completed OLE Week 5, 10, 16, 22, 34, 46, 58, 70, 82, or 94, or discontinued ozanimod treatment. aSymptomatic clinical response was defined as a decrease from baseline in the combined 6-point RBS + SFS of ≥1 point and ≥30%, and a decrease of ≥1 point in RBS or an absolute RBS ≤1 point. bSymptomatic clinical remission was defined as an RBS = 0 and SFS ≤1, and a decrease of ≥1 point from the baseline SFS. cAll patients received 52 weeks of ozanimod treatment before entering the OLE. NRI, nonresponder imputation; OC, observed case; OLE, open-label extension; RBS, rectal bleeding subscore; SFS, stool frequency subscore.
