Figure 3.
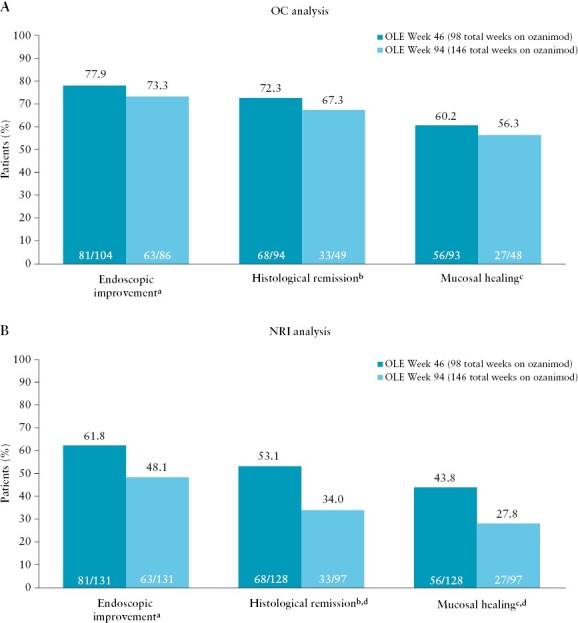
Objective outcomes [ie, endoscopic improvement, mucosal healing, and histological remission] at OLE Weeks 46 and 94 in patients on continuous ozanimod who entered the OLE in clinical response. [A] OC analysis. [B] NRI analysis. Denominators for the OC analyses were based on the numbers of patients who completed OLE Week 46 or 94 and had data available for the endpoints in question. Denominators for the NRI analyses were based on the numbers of patients who completed OLE Week 46, completed OLE Week 94, or discontinued ozanimod treatment. aEndoscopic improvement is defined as an endoscopy subscore of ≤1 point. bHistological remission is defined as a Geboes score of <2.0. cMucosal healing is defined as an endoscopy score of ≤1 point and a Geboes score of <2.0. dThree patients at OLE Week 46 and 34 patients at OLE Week 94 did not have histology data available at data cutoff and are therefore not included in the denominator for histological remission and mucosal healing. NRI, nonresponder imputation; OC, observed case; OLE, open-label extension.
