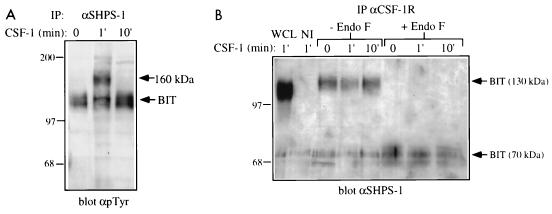
FIG. 3.
Association of BIT with the CSF-1R. (A) Transient association and phosphorylation of a ∼160-kDa protein in BIT immunoprecipitates (IP). BAC1.2F5 cells were starved of CSF-1 for 22 h and then stimulated with 2,000 U of CSF-1 per ml for the indicated times or left unstimulated (0). BIT was immunoprecipitated from these lysates by using an anti-SHPS-1 antiserum, and bound proteins were analyzed by SDS-PAGE (8% gel) and anti-pTyr immunoblotting. The migration of molecular size standards is indicated in kilodaltons at the left. (B) BIT is associated constitutively with the CSF-1R. The CSF-1R was immunoprecipitated from the same set of lysates and treated with endo F or left untreated. The 1-min lysate was also immunoprecipitated with a nonimmune serum (NI). The presence of BIT in these immunoprecipitates was assessed by immunoblotting with the anti-SHPS-1 antiserum, which is able to recognize murine BIT. Whole-cell lysate (WCL) also was tested as a positive control. The migration of molecular size standards is indicated in kilodaltons at the left.