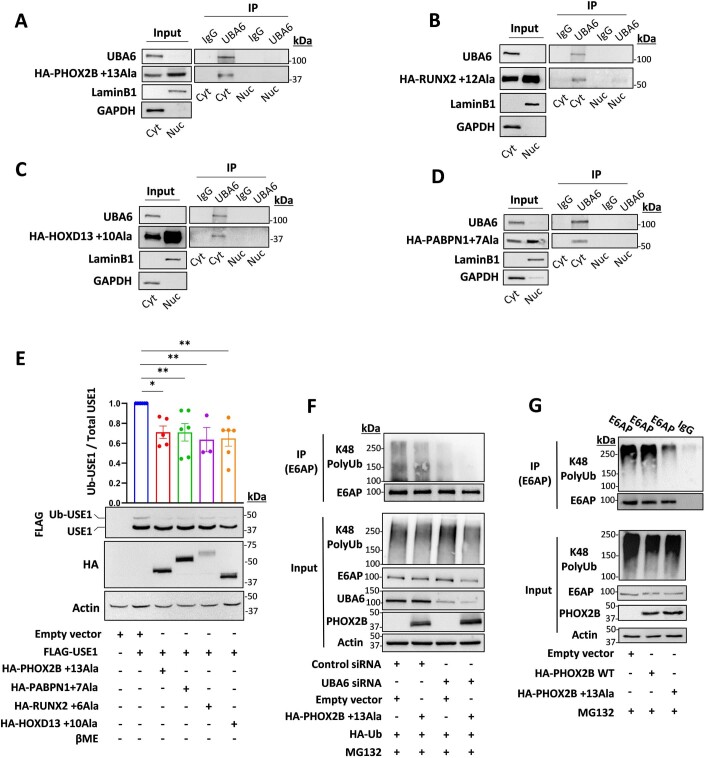
Figure EV3. Cytoplasmic polyalanine-expanded disease proteins interact with UBA6 and decrease USE1 ubiquitin loading and E6AP polyubiquitination.
(A–D) HEK293T cells were transfected with the indicated constructs: (A) HA-mutant PHOX2B (+13Ala). (B) HA-mutant RUNX2 (+12Ala). (C) HA-mutant HOXD13 (+10Ala). (D) HA-mutant PABPN1 ( + 7 Ala). Endogenous UBA6 was immunoprecipitated from the nuclear fraction (Nuc, LaminB1 enriched) or the cytoplasmic (Cyt, GAPDH enriched) fraction (unrelated IgG was used as a control). The immunocomplexes were analyzed with anti-HA antibodies. (E) HEK293T cells were transfected with constructs expressing different polyalanine-expanded disease proteins (mutant PHOX2B, mutant RUNX2, mutant HOXD13, and mutant PABPN1) together with FLAG-USE1. Cell lysates were incubated without β mercaptoethanol and analyzed for ubiquitin loading. Results are mean ± s.e.m. normalized to control (empty vector, no disease protein). Paired 2-tailed t test. n = 3–6 biological replicates. (F) Control and UBA6-depleted HEK293T cells were transfected with HA-Ub, mutant PHOX2B or empty vector and incubated for the last 6 h with the proteasome inhibitor MG132 (10 μM). Endogenous E6AP was immunoprecipitated from cell lysates for ubiquitination analysis. (G) HEK293T cells were transfected with WT PHOX2B, mutant PHOX2B or empty vector and incubated for the last 6 h with the proteasome inhibitor MG132 (10 μM). Endogenous E6AP was immunoprecipitated from cell lysates for ubiquitination analysis (unrelated IgG was used as a control). ns non-significant, *P < 0.05, **P < 0.01.