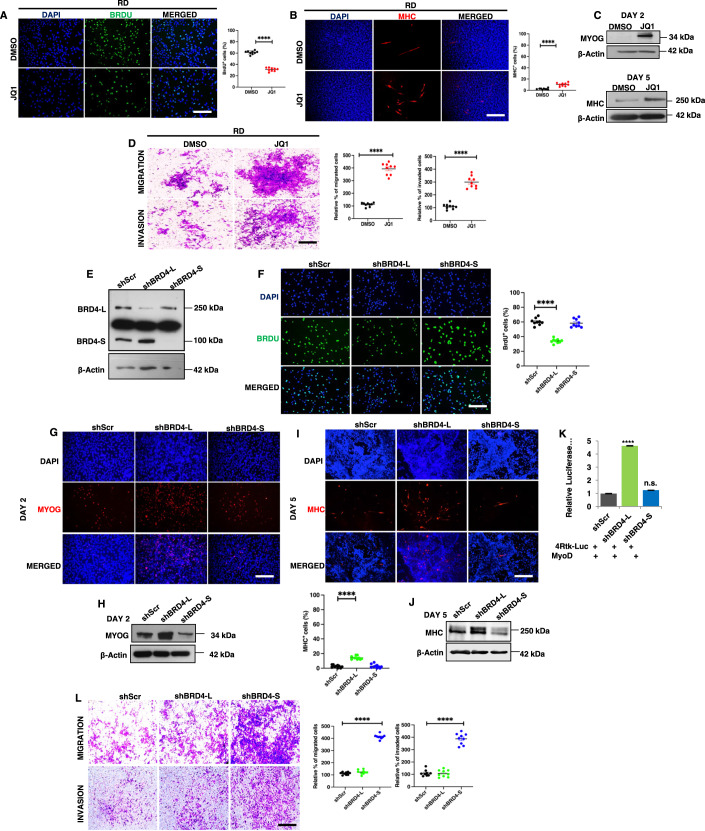
Figure 2. BRD4-L and BRD4-S have distinct roles in tumour growth and metastasis.
(A) RD cells were treated with DMSO (vehicle) or 50 nM of JQ1 for 48 h and proliferation was assessed using BrdU assay by immunofluorescence using anti-BrdU antibody. Nuclei were stained with DAPI (blue). Images are representative of at least three biological replicates. Scale bar: 100 μm. Scatter plot representing the percentage of BrdU+ cells in RD cells treated with DMSO or JQ1. Values correspond to the average ± SEM (n = 3 biological replicates with 3 technical replicates shown). Two-tailed non-parametric unpaired t test was performed for statistical analysis. ****p ≤ 0.0001. (B) RD cells were pretreated with 50 nM JQ1 in growth medium and then differentiated with DMSO or JQ1 for 5 days and analysed by immunofluorescence using anti-MHC antibody. Images are representative of at least three biological replicates. Scale bar: 100 µm. Scatter plot representing percentage of MHC+ cells in DMSO and JQ1-treated RD cells. The values correspond to average ± SEM (n = 3 biological replicates with 3 technical replicates shown). Two-tailed non-parametric unpaired t test was performed for statistical analysis. ****p ≤ 0.0001. (C) Western blot analysis using anti-MYOG antibody and anti-MHC antibody in RD cells treated with DMSO or JQ1 in differentiation media for 2 and 5 days, respectively. β-Actin was used as loading control. (D) Migratory and invasive capacity of RD cells treated with 50 nM of JQ1 for 48 h was assessed using transwell assays followed by crystal violet staining of the inserts. Images are representative of at least three biological replicates. Scale bar: 100 μm. Scatter plot representing the percentage of migration and invasion of RD cells treated with DMSO or JQ1. The values correspond to average ± SEM (n = 3 biological replicates with 3 technical replicates shown). Two-tailed non-parametric unpaired t test was performed for statistical analysis. ****p ≤ 0.0001. (E) Stable knockdown of BRD4-L (shBRD4-L) and BRD4-S (shBRD4-S) in RD cells was analysed using Western blotting. β-Actin was used as loading control. Images are representative of at least three biological replicates. (F) Proliferation was assessed using BrdU assay in shScr, shBRD4-L and shBRD4-S RD cells by immunofluorescence using anti-BrdU antibody. Nuclei were stained with DAPI. Images are representative of at least three biological replicates. Scale bar: 100 μm. Scatter plot representing the percentage of BrdU+ cells in shBRD4-L and shBRD4-S compared to shScr cells. The values correspond to average ± SEM (n = 3 biological replicates with 3 technical replicates shown). Two-tailed non-parametric unpaired t test was performed for statistical analysis. ****p ≤ 0.0001. (G) shScr, shBRD4-L and shBRD4-S cells were cultured in differentiation media for 2 days and analysed by immunofluorescence using anti-MYOG antibody. Nuclei were stained with DAPI. Images are representative of at least three biological replicates. Scale bar: 100 μm. (H) Western blot analysis of MYOG at Day 2 in shBRD4-L and shBRD4-S compared to shScr after culturing cells in differentiation media. (I) shScr, shBRD4-L and shBRD4-S cells were cultured in differentiation media for 5 days and analysed by immunofluorescence using anti-MHC antibody. Nuclei were stained with DAPI. Images are representative of at least three biological replicates. Scale bar: 100 μm. Scatter plot representing percentage of MHC+ cells in shBRD4-L and shBRD4-S cells compared to control cells. The values correspond to average ± SEM (n = 3 biological replicates with 3 technical replicates shown). Two-tailed non-parametric unpaired t test was performed for statistical analysis. ****p ≤ 0.0001. (J) Western blot analysis of MHC at Day 5 in shBRD4-L and shBRD4-S compared to shScr after culturing cells in differentiation media. β-Actin was used as loading control. (K) MyoD reporter activity was analysed in shScr, shBRD4-L and shBRD4-S cells by transfecting cells with 200 ng of the MRF reporter 4Rtk-luc, 200 ng MyoD and 5 ng Renilla. Luciferase activity was analysed 48 h post transfection. The bar graph represents the average ± SEM (n = 4 biological replicates). Two-tailed non-parametric unpaired t test was performed for statistical analysis. ****p ≤ 0.001, n.s. not significant. (L) Migratory and invasive capacity of shScr, shBRD4-L and shBRD4-S cells was assessed using transwell assays followed by crystal violet staining of the inserts. Images are representative of at least three biological replicates. Scale bar: 100 μm. Scatter plot representing the percentage of migration and invasion of shScr, shBRD4-L and shBRD4-S cells. The values correspond to average ± SEM (n = 3 biological replicates with 3 technical replicates shown). Two-tailed non-parametric unpaired t test was performed for statistical analysis. ****p ≤ 0.0001. Source data are available online for this figure.