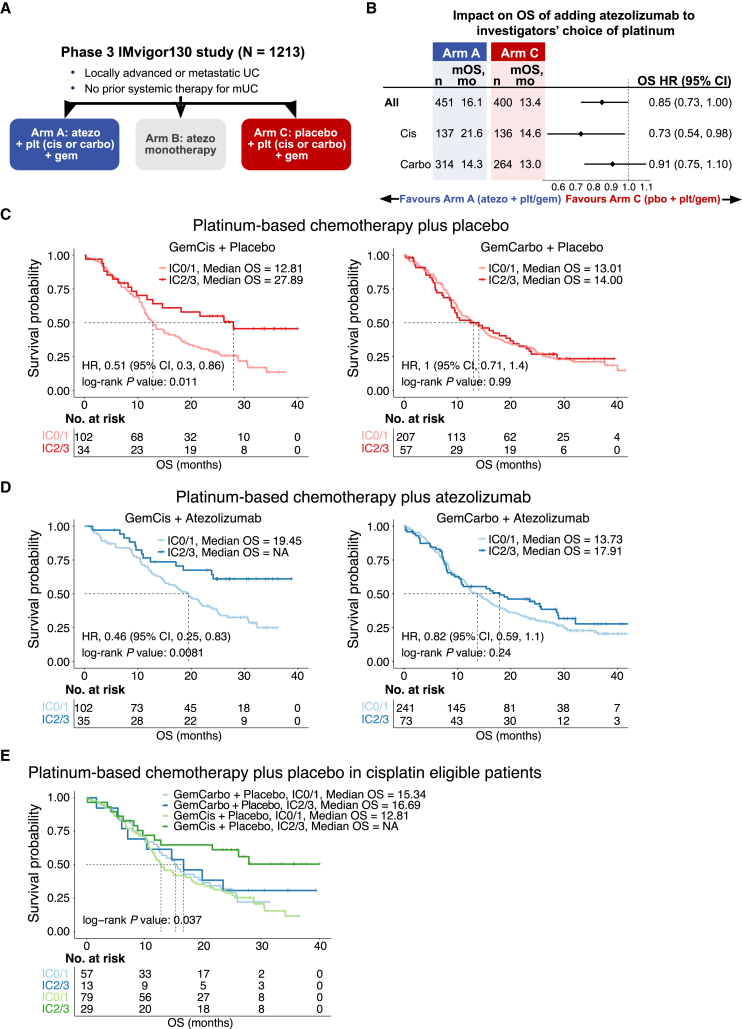
Figure 1.
Survival outcomes with GemCis but not with GemCarbo are dependent on pretreatment tumor PD-L1 expression in an exploratory analysis of the IMvigor130 study
(A) Study design of phase 3 IMvigor130. Patients in arms A and C received atezolizumab and placebo, respectively, in combination with the investigators’ choice of platinum drugs (cisplatin versus carboplatin) and gemcitabine. Arm A, n = 451; arm B, n = 362; arm C, n = 400.
(B) Forest plot showing overall survival in patients in arms A and C by use of GemCis versus GemCarbo. Hazard ratios, 95% confidence intervals, and p values were calculated using a univariate Cox model. The diamonds represent the hazard ratios, and the horizontal bars their 95% confidence intervals.
(C) Kaplan-Meier curves showing overall survival in patients in arm C stratified by PD-L1 status (IC2/3 versus IC0/1) and use of GemCis (left) or GemCarbo (right).
(D) Kaplan-Meier curves showing overall survival in patients in arm A stratified by PD-L1 status (IC2/3 versus IC0/1) and use of GemCis plus atezolizumab (left) or GemCarbo plus atezolizumab (right). In (C) and (D), p values were estimated using the log rank test. Hazard ratios and 95% confidence intervals were calculated using a univariate Cox model.
(E) Kaplan-Meier curves showing overall survival in patients in arm C stratified by actual receipt of GemCis or GemCarbo and tumor PD-L1 status (IC2/3 versus IC0/1). Patients classified as “cisplatin-eligible” according to standard criteria were included. p values were estimated using the log rank test. See also Figures S1 and S2 and Tables S1 and S2.