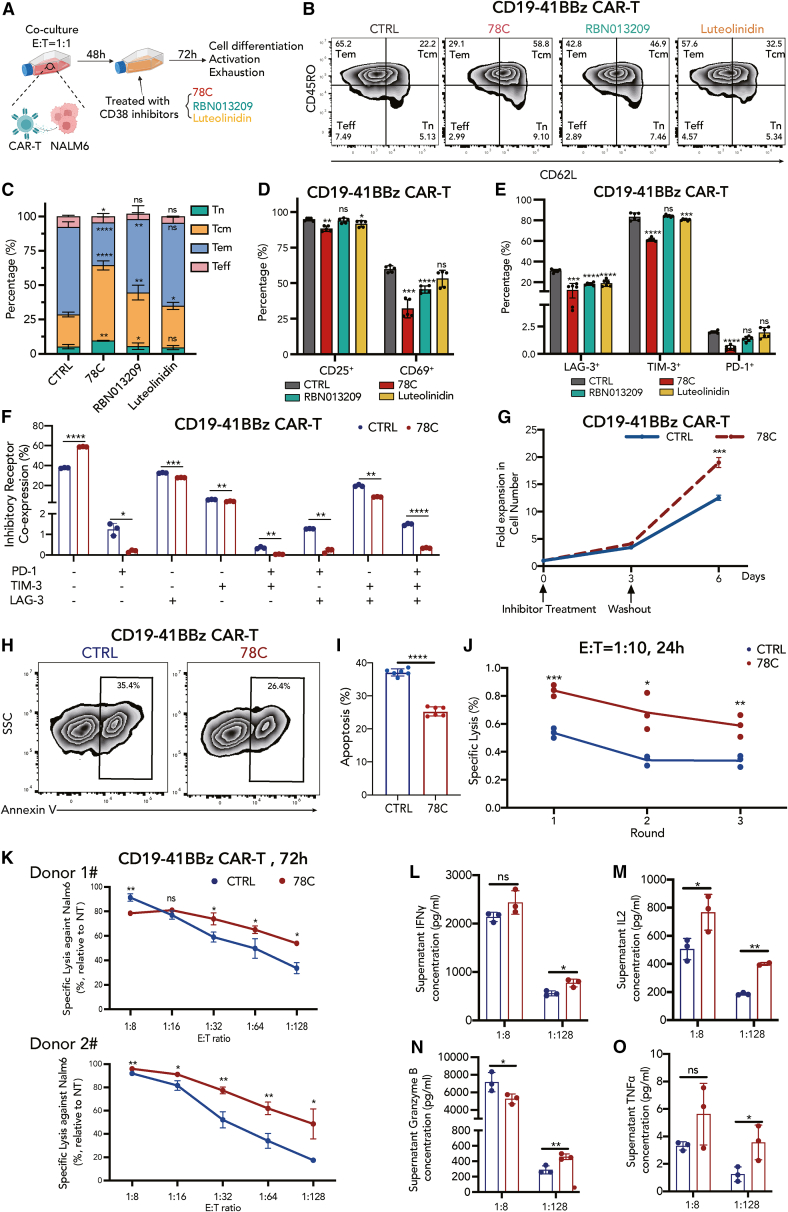
Figure 2.
CD38 inhibition promotes central memory cell formation and counteracts CAR-T cell exhaustion to enhance antitumor efficacy
(A) Schematic depicting in vitro culture model. CAR-T cells were cocultured with NALM6 at the E:T ratio of 1:1 for 48 h, followed by CD38 inhibitor treatment (78C 10 μM, RBN013209 50 μM, luteolinidin 10 μM) for 72 h. Cell differentiation, activation, and exhaustion status were evaluated by flow cytometry.
(B) Flow cytometric analysis of CD62L and CD45RO in each group.
(C) Frequency of naive cells (CD62L+, CD45RO–), central memory cells (CD62L+, CD45RO+), effector memory cells (CD62L–, CD45RO+), and effector cells (CD62L–, CD45RO–) in control or inhibitor-treated CAR-T cells 12 days after T cell activation (n = 5 biological replicates). Two-tailed Student’s unpaired t test. ∗p < 0.05, ∗∗p < 0.01, ∗∗p < 0.001, ∗∗∗∗p < 0.0001; ns, no significance. Tn, naive T; Tcm, central memory T; Tem, effector memory T; Teff, effector T. Statistical comparison is between each inhibitor-treated group with control.
(D) Frequency of CD25+ and CD69+ CAR-T cells in control or inhibitor-treated groups 12 days after T cell activation (n = 5 biological replicates).
(E) Frequency of LAG-3+, TIM-3+, and PD-1+ CAR-T cells in control or inhibitor-treated groups 12 days after T cell activation (n = 6, 3 biological replicates with two technical replicates for each donor).
(F) Frequency of inhibitory receptor co-expression (LAG-3, TIM-3, and PD-1) in control or 78C-treated groups (n = 3 biological replicates).
(G) Expansion kinetics of control and 78C-treated CD19-41BBz CAR-T cells during in vitro setting. Arrows indicate the time point of inhibitor treatment and drug washout (n = 3 technical replicates from one donor).
(H) Flow cytometric analysis of annexin V in each group.
(I) Frequency of apoptosis (annexin V+) in control or 78C-treated CAR-T cells 12 days after T cell activation (n = 6, 3 biological replicates with 2 technical replicates for each donor). Two-tailed Student’s unpaired t test.
(J) Specific lysis of NALM6-luciferase after coculture with control and 78C-treated CAR-T cells upon multiple rounds of tumor challenge. CAR-T cells were cocultured with NALM6-luci cells at the E:T ratio = 1:10 for every 24 h. Data are mean ± standard deviation (SD) of 3 technical replicates from one donor. Specific cytotoxicity is evaluated by (nontransduced T cell viability – CAR-T cell viability)/nontransduced T cell viability × 100%.
(K) Specific lysis of NALM6-luciferase after coculture with control and 78C-treated CD19-41BBz CAR-T cells for 72 h at low E:T ratio (n = 3 technical replicates from donor 1 and donor 2, respectively).
(L–O) Secretion of granzyme B, IL-2, IFNγ, and TNFα by control and 78C-treated CAR-T cells after the coculture of NALM6-luciferase for 72 h at E:T ratio of 1:1 and 1:128, respectively (n = 3 technical replicates).