Figure 3.
Perturbing CD38 boosts the efficacy of CAR-T cells against hematological malignanciesin vivo
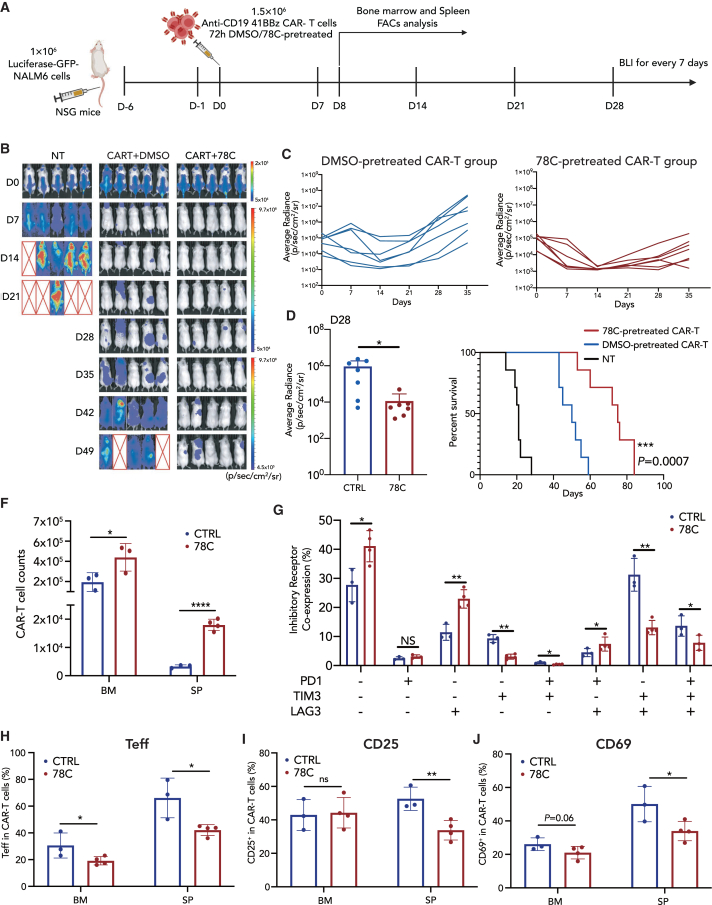
(A) Schematic depicting in vivo experimental setup. NSG mice received 1 × 106 NALM6 cells on day −6 and 1.5 × 106 either nontransduced T cells (MOCK) or 4-1BB CD19-CAR-T cells on day 0. Bone marrow and spleen tissue were collected for fluorescence-activated cell sorting analysis on day 8.
(B) D0-D49 bioluminescence imaging (BLI) imaging of tumor clearance. n = 5 biological replicates for each group.
(C) The dorsal BLI signal is displayed for individual mice in each treatment group. n = 7 biological replicates pooled from two independent experiments.
(D) BLI imaging of tumor burden on D28 after CAR-T cell infusion.
(E) Kaplan-Meier survival plot for mice receiving mock T cells, control CAR-T cells, or CAR-T cells pretreated with CD38 inhibitors. n = 7 biological replicates pooled from two independent experiments (statistical analysis by Mantel-Cox test between control CAR-T and 78C-treated CAR-T group, ∗∗∗p = 0.0007).
(F) Absolute numbers of human T cells in the bone marrow (hindlimb) and spleen on day 8 after CAR-T cell injection. n = 3 or more mice per group.
(G) Frequency of inhibitory receptor co-expression (LAG-3, TIM-3, and PD-1) in bone marrow CAR-T cells in control (n = 3 biological replicates) or 78C-treated groups (n = 4 biological replicates).
(H) Frequency of effector CAR-T cell subset (CD62L–, CD45RO–) from bone marrow and spleen tissue in control (n = 3 biological replicates) or 78C-treated groups (n = 4 biological replicates).
(I) Frequency of CD25+ and CD69+ CAR-T cells from bone marrow and spleen tissue in control (n = 3 biological replicates) or 78C-treated groups (n = 4 biological replicates).