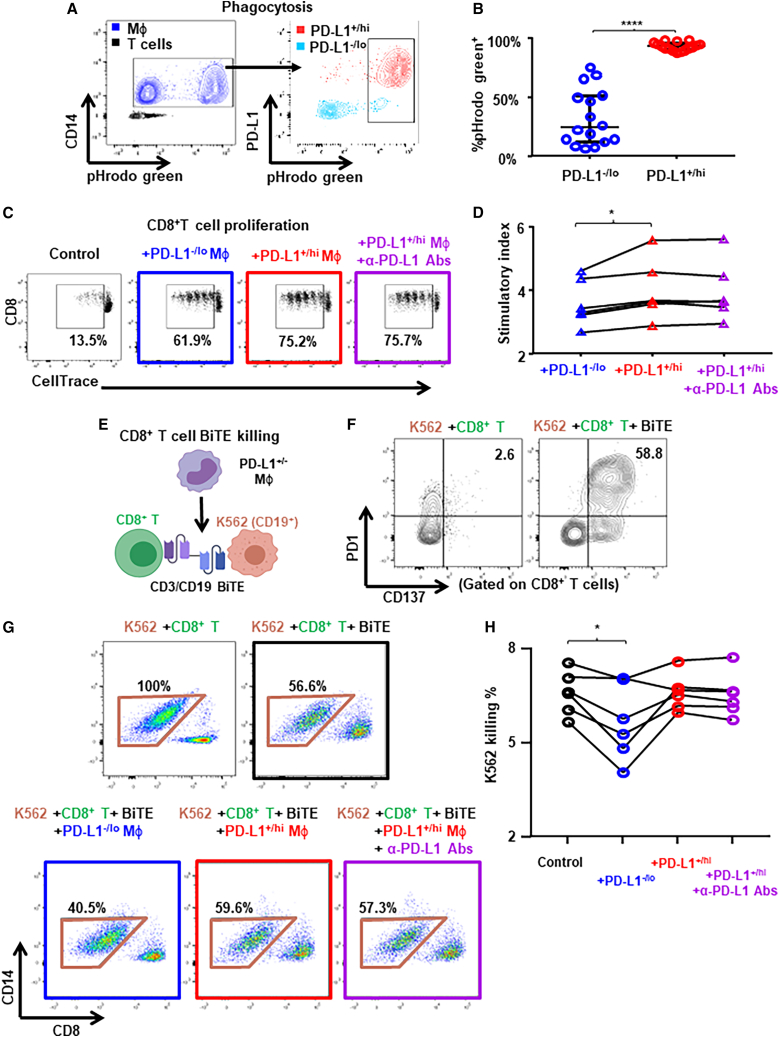
Figure 7.
PD-L1+ TAMs are more activated and pro-inflammatory than PD-L1– TAMs
(A and B) PBMCs from patients with BC (n = 16) were rested and the phagocytosis capacity of monocytes/macrophages were determined by using pHrodo Green E. Coli Bioparticles conjugate as shown in the representative flow plots (A) and compared between PD-L1–/lo vs. PD-L1+/hi monocytes/macrophages (B). Paired t test.
(C and D) Freshly isolated PBMCs from patients with BC (n = 6) were rested and the PD-L1+/− monocytes were flow sorted. CellTrace Violet dilution by CD8+ T cells determined after 4 days of TCR-stimulated coculture with autologous PD-L1+ vs. PD-L1– monocytes/macrophages. (C) Representative flow plots showing percentage of proliferated CD8+ T cells. (D) Proliferation stimulation activity measured by cell number ratio of (CD8/CD4+CD14)/(CD8/CD4) as the stimulatory index.
(E–H) Freshly isolated PBMCs from patients with BC (n = 6) were rested and the PD-L1+/− monocytes were flow sorted. Cytotoxic activity of CD8+ T cells determined using CD19/CD3 bispecific antibody (BiTE) after 2 days of coculture with CD19+ K562 cancer cells in the presence of autologous PD-L1+ or PD-L1– monocytes/macrophages. (E) Schematic representing the experiment setup. (F) Representative flow plots showing PD1 and CD137 expression on CD8+ T cells. (H) Cytotoxic activity calculated by the percentage of K562 cells killed by CD8+ T cells as shown in representative flow plots (G). One-way ANOVA. ∗p < 0.05, ∗∗∗∗p < 0.0001. Shown are mean ± SEM.