Abstract
Objectives
Although many studies have addressed such disparities caused by COVID-19, to our knowledge, no study has focused on the association of race on outcomes for patients with COVID-19 requiring venovenous extracorporeal membrane oxygenation support. The goal of this study was to assess association of race on death and duration on venovenous extracorporeal membrane oxygenation in both the pre–COVID-19 and COVID-19 eras.
Methods
We retrospectively reviewed the Extracorporeal Life Support Organization registry and included adults (≥18 years) who required venovenous extracorporeal membrane oxygenation between January 2019 and April 2021. We performed descriptive statistics and multivariable logistic regression. Our primary outcomes were death and extracorporeal membrane oxygenation duration.
Results
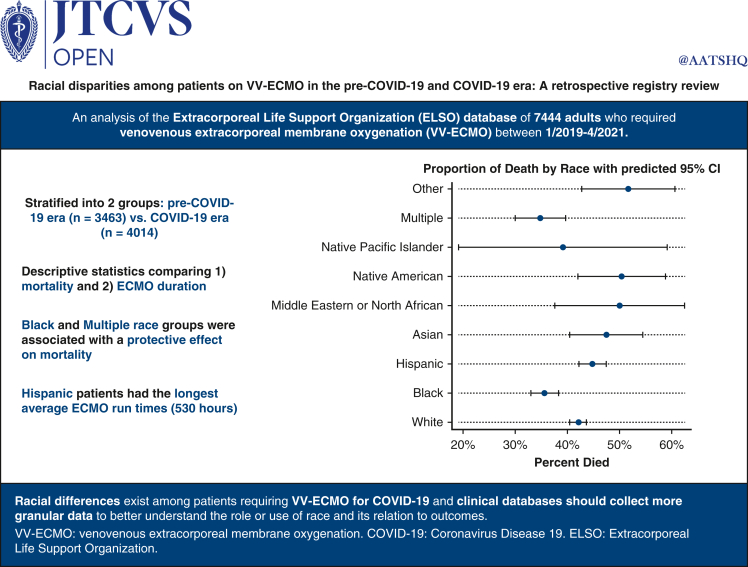
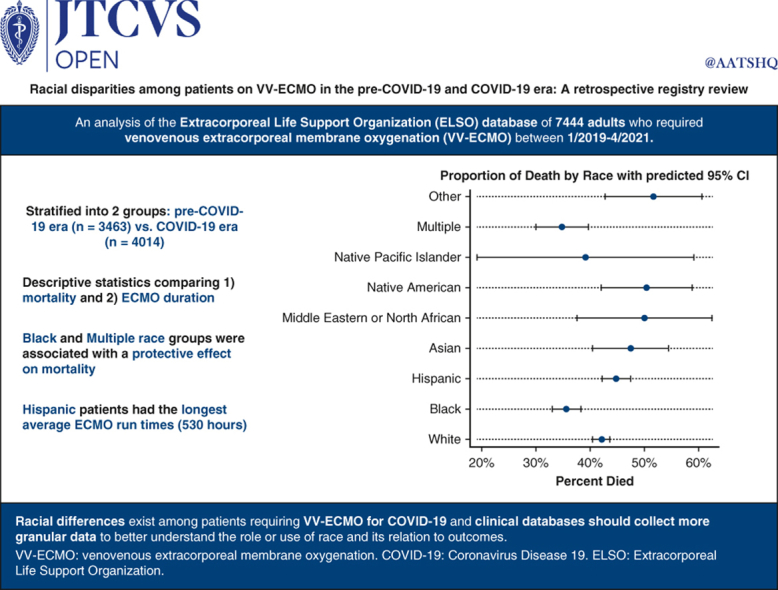
A total of 7477 patients were included after excluding 340 patients (4.3%) who were missing race data. In the COVID-19 era, 1474 of 2777 COVID-19–positive patients (53.1%) died. Our regression model suggested somewhat of a protective effect on death for Black and multiple race patients. Additionally, a diagnosis of COVID-19 and patients in the COVID-19 era in general, irrespective of COVID-19 diagnosis, had higher odds of death. Hispanic patients had the longest average venovenous extracorporeal membrane oxygenation run times.
Conclusions
Our study using data from the international Extracorporeal Life Support Organization Registry provides updated data on patients supported with venovenous extracorporeal membrane oxygenation in the pre–COVID-19 and COVID-19 eras between 2019 and 2021 with a focus on race. Patients in the COVID-19 era group also had higher mortality compared with those in the pre–COVID-19 era even after being adjusted for COVID-19 diagnosis. Black and multiple races appeared somewhat protective in terms of death. Hispanic race was associated with longer venovenous extracorporeal membrane oxygenation duration.
Key Words: COVID-19, race, racial disparities, venovenous extracorporeal membrane oxygenation, VV-ECMO
Graphical Abstract
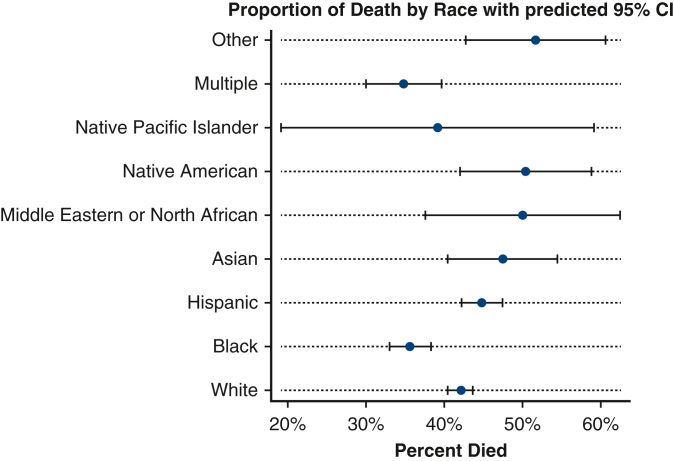
Forest plot showing the proportion of death by race with 95% CIs.
Central Message.
In an analysis of more than 7000 patients on VV-ECMO, Black and multiple race groups were associated with a protective effect on mortality, whereas Hispanic patients had the longest average ECMO duration.
Perspective.
Racial differences exist among patients requiring VV-ECMO for COVID-19. Clinical databases should collect more granular data to better understand the role or use of race and its relation to outcomes. In addition, future research might investigate better assessing and adjusting for severity of illness among patients being cannulated for VV-ECMO.
Prior research has suggested that racial disparities exist in cardiothoracic surgery, typically with Black patients having unfavorable outcomes compared with other races.1, 2, 3, 4 In an analysis of the Society of Thoracic Surgery Database of 11,697 Black and 136,362 White patients undergoing coronary artery bypass surgery, Black patients were at higher risk for postprocedural morbidity and mortality compared with White patients.1 Likewise, a single-center study of 2810 Black and 13,569 White patients who underwent coronary artery bypass graft surgery showed worse long-term survival for Black patients compared with White patients.2 A recent single-center study of 196 patients on extracorporeal membrane oxygenation (ECMO) showed that Asian, Black, and Hispanic patients were at greater risk of occult hypoxemia than White patients.4 More specifically with heart failure and respiratory failure, it has been demonstrated that there is some level of disparity in ECMO use, with the Black race being associated with higher mortality.5, 6, 7, 8 An analysis of 22,997 heart transplant recipients in the Scientific Registry of Transplant Recipients showed that Black recipients aged 18 to 30 years were at higher risk of mortality in the first year post-transplant compared with non-Black recipients.8
Despite individuals from all corners of the world being affected by COVID-19, racial disparities in the COVID-19 era persist.9, 10, 11 Many of these studies suggest that non-White patients do worse than their White counterparts with regard to mortality, but this may be due to social vulnerability and other factors related to structural racism.12,13 Still, there is a lack of data investigating racial disparities and COVID-19 within cardiothoracic surgery. One single-center study of 785 adult patients undergoing atrial fibrillation found that White patients were more likely to undergo atrial fibrillation than Black patients during the COVID-19 era with to the pre-COVID era.14 In October 2020, the international Extracorporeal Life Support Organization (ELSO) published broad findings on the outcomes of 1035 patients with COVID-19 at 213 hospitals in 36 countries who underwent cannulation for venovenous ECMO (VV-ECMO) finding that there was no significant association between race group and in-hospital mortality,15 although the number of patients treated with ECMO for COVID-19 and entered into the ELSO Registry has now increased by more than 7-fold. More recent data published by ELSO in October 2021 provided updated results on mortality but excluded race from its model in an effort to avoid “reinforcing structural inequalities.”16
Although the aforementioned data have provided much useful information on a cohort of more than 1000 COVID-19–positive patients supported on ECMO, to our knowledge, this has not been repeated among the now much larger sample size, with a focus on the association between race groups and clinical outcome differences among patients on ECMO with or without COVID-19. Likewise, we do not believe recent research has reexamined whether, particularly among a North American population of patients requiring the most advanced salvage therapy for COVID-19 VV-ECMO, such differences in outcomes exist. The goal of this study was to examine and compare racial disparities in VV-ECMO outcomes in the pre–and post–COVID-19 eras in both COVID-19–positive and non-COVID-19–positive patients. On the basis of prior literature, we hypothesized Black patients on VV-ECMO may have worse clinical outcomes compared with White patients on VV-ECMO.
Material and Methods
Data Source and Collection
We performed a retrospective review using the international ELSO ECMO Registry of patients on ECMO, which is guided by robust data definitions for data entry and whose data abstractors must pass an exam to enter data into the registry. The ELSO Registry encompasses data from more than 500 ECMO centers worldwide, including ECMO centers from the United States. We included patients who were aged 18 years or older, who received VV-ECMO support for respiratory failure, and for whom data on outcomes of death and duration on ECMO were available between January 2019 and April 2021 in ECMO centers only in North America. We defined pre–COVID-19 era as before March 11, 2020, when COVID-19 was declared a pandemic. COVID-19 era was defined as on or after March 11, 2020. Patients with VV-ECMO as their primary cannulation configuration were included in our analysis. In patients who underwent more than 1 run of VV-ECMO, we excluded details about a patient's subsequent ECMO run to reduce bias. We also excluded children (<18 years of age) and patients for whom race data were not available.
Defining Race and Racism
The ELSO Registry reports race “as determined by the patient or family” and can fall into the following categories: Asian; Black; Hispanic, Latino, or Spanish Origin; Middle Eastern or North African; Native American; Native Pacific Islander; White; Other; Unknown. The categorizations have evolved over time with the most recent change in 2017 adding Middle Eastern or North African, Native American, Native Pacific Islander, or Unknown as potential options. Additionally, the database was changed to allow multiple races to be selected rather than choosing only one. The race categories used in this study were as provided above.
Race should be understood not as a “biological construct that reflects innate differences, but a social construct that precisely captures the impacts of racism.”17 An understanding of race without an understanding of racism is problematic and has been outlined at length elsewhere.18,19 We define racism as the “differential access to the goods, services, and opportunities of society by race,” per Dr Camara Jones17 of the Centers for Disease Control and Prevention. The ELSO Registry does not capture detailed sociopolitical, economic, or cultural information for patients. Demographic information was limited to included age, sex, weight, and height for this study.
Data Analysis
The primary outcomes for this study were death and ECMO duration. Other outcomes included pH, PCO2, PO2, bicarbonate oxygen saturation, and pump flows at 4 and 24 hours of ECMO support. Baseline characteristics between groups were compared. For categorical variables, we used a chi-square test reporting global P values unless individual pairwise tests are specifically mentioned. Our primary outcome of hours on ECMO support was not normally distributed, and thus we used Wilcoxon rank-sum and Kruskal–Wallis tests. A histogram or a skewness and kurtosis test were used to assess for normality of variables. Other instances when nonparametric tests were used are indicated in the relevant tables (Table 1, Table 2, Table 3, Table 4). A 95% CI was used for statistical significance.
Table 1.
Demographic and clinical characteristics before and after March 11, 2020∗
| Variable | Pre–COVID-19 era |
COVID-19 era |
Total |
P value |
|---|---|---|---|---|
| N = 3463 | N = 4014 | N = 7477 | ||
| Age, y, mean (SD) | 47.9 (15.2) | 48.2 (13.1) | 48.0 (14.1) | .48 |
| Race | <.001 | |||
| White | 2133 (61.6%) | 1750 (43.6%) | 3883 (51.9%) | |
| Black | 568 (16.4%) | 693 (17.3%) | 1261 (16.9%) | |
| Hispanic | 346 (10.0%) | 1067 (26.6%) | 1413 (18.9%) | |
| Asian | 67 (1.9%) | 129 (3.2%) | 196 (2.6%) | |
| Middle Eastern or North African | 22 (0.6%) | 40 (1.0%) | 62 (0.8%) | |
| Native American | 45 (1.3%) | 92 (2.3%) | 137 (1.8%) | |
| Native Pacific Islander | 9 (0.3%) | 14 (0.3%) | 23 (0.3%) | |
| Multiple | 215 (6.2%) | 167 (4.2%) | 382 (5.1%) | |
| Other | 58 (1.7%) | 62 (1.5%) | 120 (1.6%) | |
| Sex | <.001 | |||
| Female | 1356 (39.2%) | 1284 (32.0%) | 2640 (35.3%) | |
| Male | 2107 (60.8%) | 2730 (68.0%) | 4837 (64.7%) | |
| Weight | 96.1 (32.6) | 98.7 (27.8) | 97.5 (30.1) | <.001 |
| Year | <.001 | |||
| 2019 | 2691 (77.7%) | 0 (0.0%) | 2691 (36.0%) | |
| 2020 | 772 (22.3%) | 3496 (87.1%) | 4268 (57.1%) | |
| 2021 | 0 (0.0%) | 518 (12.9%) | 518 (6.9%) | |
| COVID-19 positive | <.001 | |||
| No | 3461 (99.9%) | 1237 (30.8%) | 4698 (62.8%) | |
| Yes | 2 (0.1%) | 2777 (69.2%) | 2779 (37.2%) | |
| Rate | 23.4 (7.4) | 25.6 (7.1) | 24.6 (7.3) | <.001 |
| FiO2 | 93.1 (15.3) | 94.0 (13.1) | 93.6 (14.2) | .018 |
| pH | 7.25 (0.13) | 7.27 (0.13) | 7.26 (0.13) | <.001 |
| PCO2 | 63.0 (30.6) | 66.2 (30.2) | 64.8 (30.4) | <.001 |
| PO2 | 99.9 (102.2) | 89.2 (85.0) | 94.1 (93.3) | <.001 |
| HCO3 | 25.4 (6.9) | 28.0 (7.2) | 26.8 (7.2) | <.001 |
| SaO2† | 90.0 (83.0-95.0) | 91.0 (85.0-95.0) | 91.0 (84.0-95.0) | .016 |
| Renal/pulmonary/other support | 1503 (43.4%) | 2551 (63.6%) | 4054 (54.2%) | <.001 |
| Vasoactives used | 2105 (60.8%) | 2264 (56.4%) | 4369 (58.4%) | <.001 |
| Pre-ECLS arrest | <.001 | |||
| No | 3018 (87.1%) | 3725 (92.8%) | 6743 (90.2%) | |
| Unknown | 43 (1.2%) | 34 (0.8%) | 77 (1.0%) | |
| Yes | 402 (11.6%) | 255 (6.4%) | 657 (8.8%) |
FiO2, Fraction of inspired oxygen; HCO3, bicarbonate; SaO2, oxygen saturation; ECLS, extracorporeal life support.
Unless indicated as a percentage, values within parentheses represent SDs.
Nonparametric test used, and value is median with interquartile range.
Table 2.
Demographic and clinical characteristics by race group∗
| Variable | White |
Black |
Hispanic |
Asian |
Middle Eastern or North African |
Native American |
Native Pacific Islander |
Multiple |
Other |
P value |
|---|---|---|---|---|---|---|---|---|---|---|
| 3883 | 1261 | 1413 | 196 | 62 | 137 | 23 | 382 | 120 | ||
| Age (SD) | 50.3 (14.3) | 44.6 (14.3) | 45.2 (12.1) | 52.0 (14.6) | 49.3 (13.5) | 43.3 (12.2) | 40.1 (10.4) | 47.4 (14.4) | 46.4 (15.0) | <.001 |
| Sex | <.001 | |||||||||
| Female | 1390 (35.8%) | 550 (43.6%) | 380 (26.9%) | 70 (35.7%) | 13 (21.0%) | 55 (40.1%) | 8 (34.8%) | 133 (34.8%) | 41 (34.2%) | |
| Male | 2493 (64.2%) | 711 (56.4%) | 1033 (73.1%) | 126 (64.3%) | 49 (79.0%) | 82 (59.9%) | 15 (65.2%) | 249 (65.2%) | 79 (65.8%) | |
| Weight | 99.2 (29.9) | 102.8 (33.8) | 92.8 (26.8) | 78.1 (21.2) | 82.8 (18.5) | 104.7 (33.6) | 102.3 (39.0) | 92.2 (27.8) | 88.7 (27.7) | <.001 |
| Year | <.001 | |||||||||
| 2019 | 1625 (41.8%) | 448 (35.5%) | 268 (19.0%) | 52 (26.5%) | 19 (30.6%) | 34 (24.8%) | 6 (26.1%) | 186 (48.7%) | 53 (44.2%) | |
| 2020 | 1990 (51.2%) | 734 (58.2%) | 1034 (73.2%) | 126 (64.3%) | 35 (56.5%) | 94 (68.6%) | 16 (69.6%) | 182 (47.6%) | 57 (47.5%) | |
| 2021 | 268 (6.9%) | 79 (6.3%) | 111 (7.9%) | 18 (9.2%) | 8 (12.9%) | 9 (6.6%) | 1 (4.3%) | 14 (3.7%) | 10 (8.3%) | |
| COVID-19 | <.001 | |||||||||
| No | 2864 (73.8%) | 842 (66.8%) | 475 (33.6%) | 96 (49.0%) | 30 (48.4%) | 54 (39.4%) | 11 (47.8%) | 255 (66.8%) | 71 (59.2%) | |
| Yes | 1019 (26.2%) | 419 (33.2%) | 938 (66.4%) | 100 (51.0%) | 32 (51.6%) | 83 (60.6%) | 12 (52.2%) | 127 (33.2%) | 49 (40.8%) | |
| Respiratory rate | 24.1 (7.2) | 24.3 (7.6) | 26.0 (7.1) | 26.5 (7.4) | 26.9 (6.7) | 24.2 (8.4) | 23.5 (7.3) | 23.9 (7.4) | 26.3 (8.0) | <.001 |
| FiO2 | 93.5 (14.5) | 93.9 (14.7) | 93.9 (12.5) | 92.0 (15.8) | 93.0 (14.7) | 95.7 (12.4) | 89.5 (17.7) | 92.7 (14.5) | 95.9 (12.5) | .20 |
| pH | 7.26 (0.13) | 7.24 (0.14) | 7.27 (0.13) | 7.25 (0.13) | 7.24 (0.12) | 7.31 (0.13) | 7.26 (0.10) | 7.26 (0.13) | 7.24 (0.16) | <.001 |
| PCO2 | 63.5 (30.7) | 64.6 (27.3) | 65.5 (27.4) | 67.8 (30.5) | 70.5 (35.1) | 72.5 (52.4) | 70.0 (44.3) | 64.7 (26.3) | 80.4 (49.3) | <.001 |
| PO2 | 95.6 (96.7) | 90.0 (85.4) | 86.6 (76.4) | 96.0 (96.7) | 103.0 (119.5) | 129.9 (155.6) | 90.4 (118.4) | 95.0 (73.0) | 127.8 (147.5) | <.001 |
| HCO3 | 26.4 (7.1) | 25.9 (6.8) | 28.5 (7.3) | 27.4 (7.7) | 27.6 (9.3) | 27.8 (7.7) | 25.9 (7.0) | 27.2 (6.8) | 27.8 (8.3) | <.001 |
| SaO2† | 90.0 (84.0-95.0) | 90.0 (82.0-95.0) | 91.0 (86.0-95.0) | 92.0 (87.0-96.0) | 91.0 (82.0-95.0) | 90.0 (85.0-95.0) | 92.0 (89.0-95.0) | 92.0 (86.0-96.0) | 90.0 (84.0-96.0) | .066 |
| Renal/pulmonary/other support | 2003 (51.6%) | 637 (50.5%) | 888 (62.8%) | 125 (63.8%) | 39 (62.9%) | 103 (75.2%) | 15 (65.2%) | 182 (47.6%) | 62 (51.7%) | <.001 |
| Vasoactives used | 2305 (59.4%) | 737 (58.4%) | 798 (56.5%) | 135 (68.9%) | 36 (58.1%) | 69 (50.4%) | 14 (60.9%) | 201 (52.6%) | 74 (61.7%) | .005 |
| Pre-ECLS arrest | <.001 | |||||||||
| No | 3481 (89.6%) | 1102 (87.4%) | 1336 (94.6%) | 177 (90.3%) | 52 (83.9%) | 133 (97.1%) | 20 (87.0%) | 333 (87.2%) | 109 (90.8%) | |
| Unknown | 34 (0.9%) | 15 (1.2%) | 13 (0.9%) | 1 (0.5%) | 1 (1.6%) | 0 (0.0%) | 0 (0.0%) | 13 (3.4%) | 0 (0.0%) | |
| Yes | 368 (9.5%) | 144 (11.4%) | 64 (4.5%) | 18 (9.2%) | 9 (14.5%) | 4 (2.9%) | 3 (13.0%) | 36 (9.4%) | 11 (9.2%) |
FiO2, Fraction of inspired oxygen; HCO3, bicarbonate; SaO2, oxygen saturation; ECLS, extracorporeal life support.
Unless indicated as a percentage, values within parentheses represent SDs.
Nonparametric test used, and value is median with interquartile range.
Table 3.
Outcomes by pre–post status∗
| Variable | Pre–COVID-19 era |
COVID-19 era |
Total |
P value |
|---|---|---|---|---|
| N = 3463 | N = 4014 | N = 7477 | ||
| pH at 24 h | 7.40 (0.07) | 7.40 (0.07) | 7.40 (0.07) | .85 |
| PCO2 at 24 h | 44.7 (24.8) | 48.6 (27.2) | 46.9 (26.2) | <.001 |
| PO2 at 24 h† | 85.0 (68.0-119.0) | 78.0 (65.0-102.0) | 80.9 (66.0-110.0) | <.001 |
| HCO3 at 24 h | 25.7 (5.3) | 27.6 (5.7) | 26.7 (5.6) | <.001 |
| SaO2 at 24 h† | 96.0 (93.0-98.0) | 95.0 (92.0-97.0) | 95.0 (92.0-98.0) | <.001 |
| Pump flow at 4 h | 4.1 (0.9) | 4.3 (0.8) | 4.2 (0.9) | <.001 |
| Pump flow at 24 h | 4.2 (0.9) | 4.3 (0.9) | 4.3 (0.9) | <.001 |
| Hours on ECMO | 174.0 (90.0-336.0) | 329.0 (143.0-627.0) | 238.0 (113.0-494.0) | <.001 |
| Died | 1191 (34.4%) | 1921 (47.9%) | 3112 (41.6%) | <.001 |
HCO3, Bicarbonate; SaO2, oxygen saturation; ECMO, extracorporeal membrane oxygenation.
Unless indicated as a percentage, values within parentheses represent SDs.
Nonparametric test used, and value is median with interquartile range.
Table 4.
Outcomes by race group∗
| Variable | White |
Black |
Hispanic |
Asian |
Middle Eastern or North African |
Native American |
Native Pacific Islander |
Multiple |
Other |
P value |
|---|---|---|---|---|---|---|---|---|---|---|
| 3883 | 1261 | 1413 | 196 | 62 | 137 | 23 | 382 | 120 | ||
| pH at 24 h | 7.40 (0.07) | 7.39 (0.07) | 7.40 (0.07) | 7.40 (0.07) | 7.39 (0.07) | 7.41 (0.05) | 7.38 (0.08) | 7.40 (0.07) | 7.39 (0.07) | .004 |
| PCO2 at 24 h | 46.6 (26.7) | 44.9 (21.5) | 47.9 (23.7) | 46.3 (27.0) | 47.6 (28.6) | 58.6 (51.6) | 43.5 (9.1) | 45.2 (17.2) | 56.5 (46.4) | <.001 |
| PO2 at 24 h† | 81.6 (67.0-112.0) | 83.0 (68.0-115.0) | 76.0 (63.0-99.0) | 82.0 (66.0-121.0) | 87.0 (63.0-123.0) | 72.0 (62.0-104.0) | 80.0 (71.6-112.8) | 85.0 (70.0-113.0) | 83.0 (64.0-101.2) | <.001 |
| HCO3 at 24 h | 26.5 (5.6) | 25.9 (5.1) | 27.9 (5.7) | 26.7 (5.3) | 27.1 (5.9) | 28.2 (6.3) | 25.0 (5.3) | 26.7 (5.3) | 27.1 (5.4) | <.001 |
| SaO2 at 24 h† | 96.0 (93.0-98.0) | 96.0 (92.0-98.0) | 95.0 (91.0-97.0) | 95.0 (92.0-98.0) | 96.0 (92.0-98.0) | 94.0 (91.0-96.0) | 96.0 (93.0-97.0) | 96.0 (93.0-97.0) | 95.5 (92.0-97.0) | <.001 |
| Pump flow at 4 h | 4.2 (0.9) | 4.2 (0.9) | 4.2 (0.8) | 3.9 (0.7) | 4.1 (0.7) | 4.5 (0.9) | 4.3 (1.2) | 4.1 (0.8) | 4.1 (1.1) | <.001 |
| Pump flow at 24 h | 4.3 (0.9) | 4.3 (0.9) | 4.3 (0.9) | 4.0 (0.7) | 4.1 (0.8) | 4.5 (1.0) | 4.2 (0.8) | 4.2 (0.8) | 4.1 (1.1) | <.001 |
| Hours on ECMO† | 209.0 (96.0-420.0) | 221.0 (113.0-449.0) | 365.0 (165.0-691.0) | 319.0 (141.0-613.0) | 308.0 (141.0-632.0) | 329.0 (160.0-625.0) | 213.0 (94.0-528.0) | 217.5 (89.0-459.0) | 286.5 (120.0-568.5) | <.001 |
| Died | 1633 (42.1%) | 449 (35.6%) | 633 (44.8%) | 93 (47.4%) | 31 (50.0%) | 69 (50.4%) | 9 (39.1%) | 133 (34.8%) | 62 (51.7%) | <.001 |
ECMO, Extracorporeal membrane oxygenation; HCO3, bicarbonate; SaO2, oxygen saturation.
Unless indicated as a percentage, values within parentheses represent SDs.
Nonparametric test used, and value is median with interquartile range.
We used a multivariable model to assess the association between race group and outcomes. The model was informed by using both statistical criteria (eg, significant associations on univariable analysis) and what we deemed important clinical criteria with face validity. Our final multivariable model included age, sex, race group, pre-COVID versus COVID-19 era status, pre-ECLS arrest, and several hemodynamic or metabolic parameters before ECMO support including respiratory rate, fraction of inspired oxygen, pH, pCO2, PO2, bicarbonate, oxygen saturation (SaO2), and COVID-19 positivity.13,14 Missing data were excluded for individual analyses. Adjusted odds ratios (aORs) with 95% CIs are presented.
Ethical Approval
We applied for ethical approval through the Johns Hopkins Medicine Institutional Review Board (IRB00280941), and the research was determined to be exempt under Department of Health and Human Services regulations on March 23, 2021.
Results
Overall Demographic and Clinical Profile
A total of 7477 patients were included in our study after excluding 340 patients (4.3%) who did not have data on race available. Overall, our sample included 2640 women (35.3%) and 4837 men (64.7%) with a mean age of 48.0 years (SD = 14.1). A total of 3883 patients (51.9%) were White, 1261 (16.9%) were Black, 1413 (18.9%) were Hispanic, 196 (2.6%) were Asian, 62 (0.8%) were Middle Eastern or North African, 137 (1.8%) were Native American, 23 (0.3%) were Native Pacific Islander, 382 (5.1%) were multiple races, and 120 (1.6%) were Other. Among all patients included in the study, 2779 (37.2%) had COVID-19 disease.
Differences between the pre- and post-groups were significant for several variables (Table 1). Notably, patients in the COVID-19 era were more frequently male than in the pre–COVID-19 era (COVID-19 era: n = 2730, 68.0% vs pre–COVID-19 era: n = 2107, 60.8%, P < .001). Most patients in the COVID-19 era were COVID-19 positive (n = 2777, 69.2%). Importantly when taken together, there was a significant difference in the race breakdown of the pre–COVID-19 era and the COVID-19 era with fewer patients in the COVID-19 era group being White, Multiple, and Other race categories, and more patients being Black, Hispanic, Asian, Middle Eastern or North African, Native American, and Native Pacific Islander (P < .0001, Table 1). Patients in the COVID-19 era group had a lower pO2 than patients in the pre–COVID-19 era group (pO2 = 89.2 vs 99.9, P < .001).
Demographic and Clinical Profile by Race Category
Hispanic patients were more frequently COVID-19 positive (n = 938, 66.4%) followed by Native American patients (n = 83, 60.6%), and White patients were least frequently COVID-19 positive (n = 1019, 26.2%, P < .001, Table 2). Before ECMO cannulation, Hispanic and Black patients had the lowest pO2 compared with all other race groups: Hispanic pO2: 86.6; Black pO2: 90.0 (P < .001). There were no significant differences in fraction of inspired oxygen among the race groups (P = .20).
Outcomes and On-Extracorporeal Membrane Oxygenation Variables
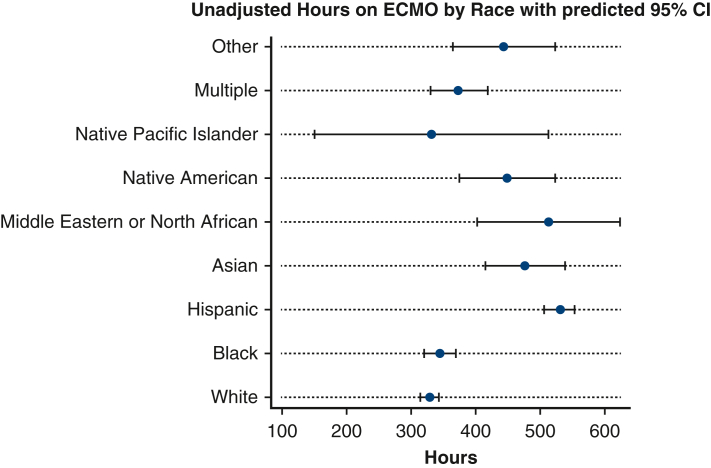
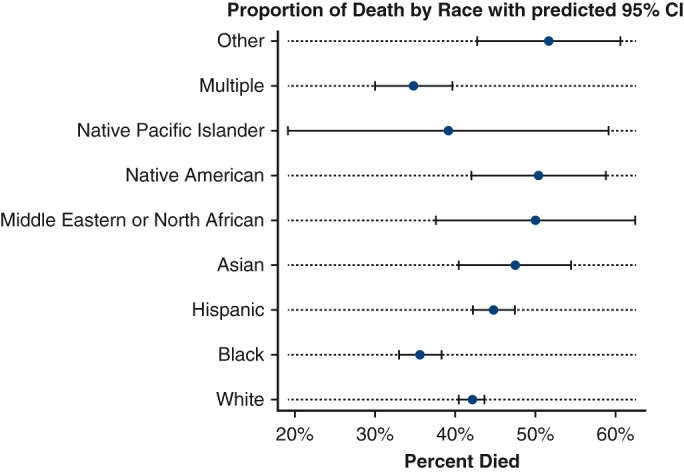
Patients in the post–COVID-19 era group were more likely to have a lower PO2 at 24 hours of ECMO support (78.0 vs 85.0, P < .001). Additionally, patients in the COVID-19 era group were more likely to die (n = 1,921, 47.9%) when compared with patients in the pre–COVID-19 era group (n = 1191, 34.4%, Figure 1). Patients in the COVID-19 era group were also more likely to have significantly longer run-times on ECMO (329 hours) when compared with the pre–COVID-19 era group (174.0 hours, P < .001, Table 3 and Figure 2). In the post–March 11, 2020, group, 1474 of 2777 COVID-19–positive patients (53.1%) died.
Figure 1.
Forest plot showing the proportion of death by race with predicted 95% CIs.
Figure 2.
Forest plot showing the number of unadjusted hours on ECMO by race with predicted 95% CIs. ECMO, Extracorporeal membrane oxygenation.
Compared with all other groups, Hispanic patients had the lowest PO2 at 24 hours (76.0) (P < .001, Table 4). Hispanic patients had the longest ECMO run times (median: 365 hours), followed by Native American patients (median: 329 hours) when compared with all groups (P < .001). Finally, Black patients (n = 449, 35.6%) and Multiple race patients (n = 133, 34.8%) were the least likely to die when compared with all groups, whereas Other race (n = 62, 51.7%), Middle Eastern or North African (n = 31, 50.0%), and Native American (n = 69, 50.4%) were the most likely to die (P < .001, Table 4).
Multivariable Regression Analyses
On multivariable analysis adjusted for covariates in the model and as listed in Table 5, Black patients (aOR, 0.78; 95% CI, 0.65-0.93) and Multiple Race group patients (aOR, 0.68, 95% CI, 0.49-0.93) had significantly lower odds of death when compared with White patients. Patients with a diagnosis of COVID-19 had 81% higher odds of death compared with patients without COVID (aOR, 1.81, 95% CI, 1.49-2.19). Patients in the COVID-19 era group had higher odds of death when compared with patients in the pre–COVID-19 era group (aOR, 1.31; 95% CI, 1.09-1.57). No significant sex differences were observed in the multivariable model (Table 5). When compared with White patients, total hours on ECMO were significantly higher in Hispanic patients (67.1 hours longer, 95% CI, 33.3-100.8 hours). No other significant differences in ECMO run times were observed with respect to race in our multivariable model. Patients with COVID-19 had significantly longer run times (239.0 hours longer, 95% CI, 201.4-276.7 hours) than patients without COVID-19.
Table 5.
Multivariable logistic regression model for death
| Variable | Odds ratio | 95% CI |
|---|---|---|
| Pre–COVID-19 era or COVID-19 era | 1.31 | 1.09-1.57 |
| Race | ||
| Black | 0.78 | 0.65-0.93 |
| Hispanic | 0.96 | 0.80-1.14 |
| Asian | 0.80 | 0.54-1.18 |
| Middle Eastern or North African | 1.05 | 0.55-1.99 |
| Native American | 1.39 | 0.85-2.27 |
| Native Pacific Islander | 0.49 | 0.15-1.60 |
| Multiple | 0.68 | 0.49-0.93 |
| Other | 1.45 | 0.90-2.33 |
| Age | 1.04 | 1.03-1.04 |
| Sex | ||
| Male | 1.01 | 0.88-1.15 |
| FiO2 | 1.00 | 1.00-1.01 |
| pH | 0.11 | 0.05-0.23 |
| PCO2 | 1.00 | 0.99-1.00 |
| PO2 | 1.00 | 1.00-1.00 |
| HCO3 | 1.02 | 1.00-1.03 |
| SaO2 | 0.99 | 0.98-1.00 |
| Pre-ECLS arrest | 1.51 | 1.21-1.88 |
| COVID-19 positive | 1.81 | 1.49-2.19 |
FiO2, Fraction of inspired oxygen; HCO3, bicarbonate; SaO2, oxygen saturation; ECLS, extracorporeal life support.
Discussion
Our study using data from the international ELSO Registry provides updated data on patients supported with VV-ECMO in the pre–COVID-19 and COVID-19 eras between 2019 and 2021 (Figure 3). Our data suggest that overall, most patients in the post–March 11, 2020, period were on VV-ECMO for a diagnosis of COVID-19. Patients in the COVID-19 era group also had higher mortality compared with those in the pre–March 11, 2020, era even after being adjusted for COVID-19 diagnosis. According to publicly available US census data, our study population was underrepresented regarding White and Asian people, slightly overrepresented regarding Black people, and had about the same proportion of Hispanics, Native Pacific Islanders, and Native Americans, as is in the population of the United States.
Figure 3.
Summary of our key findings. Using data from the ELSO Registry, overall, most patients in the post–March 11, 2020, period were on VV-ECMO for a diagnosis of COVID-19. Patients in the COVID-19 era group also had higher mortality compared with those in the pre–March 11, 2020, era even after being adjusted for COVID-19 diagnosis.
Previous research on the association between COVID-19 and death in a VV-ECMO–supported population suggests that mortality is similar in COVID-19 and non–COVID-19 ECMO-supported patients.20 Although not as high as some of the earliest estimates of COVID-19 mortality for patients supported on VV-ECMO, our analysis of more than 2700 patients with COVID-19 suggests that the mortality rate is over 50% for COVID-19–positive patients.15 Our multivariable model, adjusting for several demographic and clinically relevant factors, also showed 81% higher odds of death in COVID-19–positive versus negative patients.
To our knowledge, we present the largest and most granular breakdown of race groups for patients supported with VV-ECMO with and without COVID-19. Although our study design was unable to capture the true denominator of patients considered for ECMO support, we did appreciate a substantial increase of Hispanic patients in the post–March 11, 2020, era, whereas the proportion of White patients decreased. This is consistent with several studies documenting disproportionate effects on COVID-19 among Hispanic populations in the United States.10,21,22 Our study builds on prior studies by including a population receiving VV-ECMO, the highest level of support for COVID-19. We did not observe any differences with respect to odds of death and race when compared with White patients. Interestingly, it appears Black and Multiple races conferred a mild to moderate reduced odds of death.
Of note, our description captures the race group of a patient, but in and of itself, race is a social construct that is often used as a surrogate for other covariates (eg, comorbidities, education, occupation, health behavior, socioeconomic status). A plethora of scholarship demonstrates that social factors for which race is being used as a surrogate affect health outcomes.17,23 More recently, racism has been aptly implicated in poor health outcomes, including in the COVID-19 population.13,18 Although race is not used as a specific criterion in international guidelines for ECMO initiation, the effects of racism are apparent when one considers the relative contraindications in selection, such as preexisting conditions and end-stage malignancy. The triaging of which patients to select for ECMO must consider how nonclinical factors may affect the clinical eligibility of a patient; otherwise, equity concerns arise over particular inclusion and exclusion models. For example, in many studies, Black race is associated with an increased odds of death and may deter clinicians from pursuing aggressive clinical care, and recent data have suggested that there are disparities in selection for scarce resources such as lung transplantation.24,25 In our study, Black and Multiple race patients were associated with a decreased odds of death. In our adjusted multivariable logistic regression model, we observed other independent risk factors of increased odds of death such as positive COVID-19 status and the presence of pre-ECMO cardiac arrest. These factors may drive the mortality risk in patients on VV-ECMO more than the race group itself and thus give one explanation of our findings. Furthermore, Black patients were also of younger age and had more normal arterial blood gas values, relative to other race groups, which may reflect a lower severity of illness at the start of ECMO cannulation and thus may partially explain our findings. Nevertheless, race in of itself does not explain these disparate physiological findings, and future research with more granular data is required to fully understand the mechanisms driving these disparities.
Hispanic patients on average had the longest ECMO median run times. COVID-19 has brought renewed attention to understanding and evaluating the use and distribution of limited resources in the context of a pandemic or crisis.26, 27, 28 Although our findings do suggest that Hispanic patients on average had significantly longer ECMO run times, we caution against this being used as a relative contraindication and potential bias for not placing a Hispanic patient on ECMO given the obvious equity concern and the ways in which particular communities have already been disproportionately affected by the COVID-19 pandemic.24,29 Furthermore, this longer duration of ECMO support for Hispanic patients may reflect a greater severity of illness of this group at the start of ECMO cannulation, but it is difficult to determine underlying factors given a lack of granular data regarding socioeconomic factors.
Limitations
Our study had several limitations including its retrospective design, obvious constraints during the pandemic, and potential for absent data. Although we did assess differences in demographic profiles and outcomes in the pre–COVID-19 versus COVID-19 era groups, we were unable to assess more granular changes and trends (eg, interrupted time series modeling) because data were not available by month or day. This limited our ability to perform, for example, a difference-in-difference model to assess both overall changes and changes in the rate in which certain groups of patients may have presented. Regarding our multivariable model, there could be spurious associations detected given the hemodynamic parameters used in our model. Although not representing a large proportion of patients, patients whose race category was Multiple may have fallen into more than 1 of the other groups. In the ELSO Registry, this is not provided as individual, binary variables for each race category for each patient, but rather as a conglomerate. It would aid future research to provide this as binary variables for each race category so it could be determined which races comprise a “Multiple” race identity for individual patients. Likewise, race and ethnicity, particularly for patients who were Hispanic/Latino in our study, were collapsed into a single group per the ELSO data dictionary definitions, despite these inherent choices representing different races and ethnicities. Future database revisions might consider adding a separate variable for ethnicity in addition to race. Regarding COVID-19 positivity, our study design assumed a negative finding if a patient had not been tested given the high rates of COVID testing in the current pandemic, and we assumed most patients on VV-ECMO in the current era would have been tested for COVID-19. Likewise, patient groups for this study were defined not primarily by the underlying reason they received VV-ECMO support but rather by date of cannulation (eg, before or after March 11, 2020). This could introduce bias in our sample. Additionally, we were unable to determine how long after symptom presentation that patients were required or offered to be put on ECMO support, which likely influences clinical outcomes as well. We were also unable to analyze how disparities changed between different waves of the pandemic and how vaccination rates may have differed between race groups in this Registry. Additionally, the ELSO Registry does not contain granular and specific data to define health disparities, including but not limited to variables like median household income based on ZIP code, disparity indices, urban versus rural hospital center, insurance coverage, access to health care, whether patients experienced stress or racism, and more. We were also not able to compare single payor versus multipayer medical insurance models, because the ELSO Registry does not collect these data. Such variables need to be better collected to better investigate the effect of race on health disparities within patients on ECMO. Finally, because of the lack of granularity in the ELSO Registry for variables defining acuity of illness and comorbidities, we were unable to determine if differences in race outcomes simply reflected pathophysiological differences between each race group. For example, duration of intubation before ECMO, associated bacterial infections such as pneumonia, creatinine level to assess renal function, and therapies received for COVID-19 would be strong proxies to evaluate a patient's severity of illness.
Conclusions
Our study sheds light on the importance of expanding prospective clinical databases to include more granular data for which race is being used as a surrogate. Additional data on median household income, education, cultural factors, health behavior, socioeconomic status, and occupation would aid in better understanding the social context of individual patients rather than using race in its place. In addition, future research might investigate better assessing and adjusting for severity of illness among patients being cannulated for VV-ECMO.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Acknowledgments
The authors thank the ELSO for granting the data request for this study. The data were acquired after formal application to ELSO, which approved our request to obtain deidentified data.
Footnotes
Institutional Review Board Approval: We applied for ethical approval through to the Johns Hopkins Medicine Institutional Review Board (#00280941), and the research was determined to be exempt under Department of Health and Human Services regulations on March 23, 2021. All procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975.
References
- 1.Mehta R.H., Shahian D.M., Sheng S., O'Brien S.M., Edwards F.H., Jacobs J.P., et al. Association of hospital and physician characteristics and care processes with racial disparities in procedural outcomes among contemporary patients undergoing coronary artery bypass grafting surgery. Circulation. 2016;133:124–130. doi: 10.1161/CIRCULATIONAHA.115.015957. [DOI] [PubMed] [Google Scholar]
- 2.Keeling W.B., Binongo J., Halkos M.E., Leshnower B.G., Nguyen D.Q., Chen E.P., et al. The racial paradox in multiarterial conduit utilization for coronary artery bypass grafting. Ann Thorac Surg. 2017;103:1214–1221. doi: 10.1016/j.athoracsur.2016.07.042. [DOI] [PubMed] [Google Scholar]
- 3.Hartz R.S., Rao A.V., Plomondon M.E., Grover F.L., Shroyer A.L. Effects of race, with or without gender, on operative mortality after coronary artery bypass grafting: a study using the Society of Thoracic Surgeons National Database. Ann Thorac Surg. 2001;71:512–520. doi: 10.1016/s0003-4975(00)02030-0. [DOI] [PubMed] [Google Scholar]
- 4.Kalra A., Shou B.L., Zhao D., Wilcox C., Keller S.P., Whitman G.J., et al. Racial and ethnical discrepancy in hypoxemia detection in patients on extracorporeal membrane oxygenation. JTCVS Open. 2023;14:145–170. doi: 10.1016/j.xjon.2023.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arora A., Tsangaris A., Amin A., Song D., Agrawal P., Hajjar R., et al. Regional disparities and national trends in mortality rates among cardiac arrest patients managed with extracorporeal membrane oxygenation. J Am Coll Cardiol. 2018;71:A1313. [Google Scholar]
- 6.Chan T., Barrett C.S., Tjoeng Y.L., Wilkes J., Bratton S.L., Thiagarajan R.R. Racial variations in extracorporeal membrane oxygenation use following congenital heart surgery. J Thorac Cardiovasc Surg. 2018;156:306–315. doi: 10.1016/j.jtcvs.2018.02.103. [DOI] [PubMed] [Google Scholar]
- 7.Erickson S.E., Shlipak M.G., Martin G.S., Wheeler A.P., Ancukiewicz M., Matthay M.A., et al. Racial and ethnic disparities in mortality from acute lung injury. Crit Care Med. 2009;37:1–6. doi: 10.1097/CCM.0b013e31819292ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maredia H., Bowring M.G., Massie A.B., Bae S., Kernodle A., Oyetunji S., et al. Better understanding the disparity associated with black race in heart transplant outcomes: a national registry analysis. Circ Heart Fail. 2021;14 doi: 10.1161/CIRCHEARTFAILURE.119.006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez L., III, Hart L.H., III, Katz M.H. Racial and ethnic health disparities related to COVID-19. JAMA. 2021;325:719–720. doi: 10.1001/jama.2020.26443. [DOI] [PubMed] [Google Scholar]
- 10.Webb Hooper M., Nápoles A.M., Pérez-Stable E.J. COVID-19 and racial/ethnic disparities. JAMA. 2020;323:2466–2467. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chowkwanyun M., Reed A.L. Racial health disparities and covid-19 — caution and context. N Engl J Med. 2020;383:201–203. doi: 10.1056/NEJMp2012910. [DOI] [PubMed] [Google Scholar]
- 12.Price-Haywood E.G., Burton J., Fort D., Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382:2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegel M., Critchfield-Jain I., Boykin M., Owens A., Nunn T., Muratore R. Actual racial/ethnic disparities in COVID-19 mortality for the non-Hispanic Black compared to non-Hispanic White population in 353 US counties and their association with structural racism. J Racial Ethn Health Disparities. 2022;9:1697–1725. doi: 10.1007/s40615-021-01109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tien M., Saddic L.A., Neelankavil J.P., Shemin R.J., Williams T.M. The impact of COVID-19 on racial and ethnic disparities in cardiac procedural care. J Cardiothorac Vasc Anesth. 2023;37:732–747. doi: 10.1053/j.jvca.2023.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbaro R.P., MacLaren G., Boonstra P.S., Iwashyna T.J., Slutsky A.S., Fan E., et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396:1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbaro R.P., MacLaren G., Boonstra P.S., Combes A., Agerstrand C., Annich G., et al. Extracorporeal membrane oxygenation for COVID-19: evolving outcomes from the international extracorporeal Life support Organization registry. Lancet. 2021;398:1230–1238. doi: 10.1016/S0140-6736(21)01960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones C.P. Levels of racism: a theoretic framework and a gardener's tale. Am J Public Health. 2000;90:1212–1215. doi: 10.2105/ajph.90.8.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khazanchi R., Evans C.T., Marcelin J.R. Racism, not race, drives inequity across the COVID-19 continuum. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.19933. [DOI] [PubMed] [Google Scholar]
- 19.Boyd R.W., Lindo E.G., Weeks L.D., Mclemore M.R. On racism: a new standard for publishing on racial health inequities. 2020. [Google Scholar]
- 20.Combes A., Hajage D., Capellier G., Demoule A., Lavoué S., Guervilly C., et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 21.Buikema A.R., Buzinec P., Paudel M.L., Andrade K., Johnson J.C., Edmonds Y.M., et al. Racial and ethnic disparity in clinical outcomes among patients with confirmed COVID-19 infection in a large US electronic health record database. EClinicalMedicine. 2021;39 doi: 10.1016/j.eclinm.2021.101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Disparities in COVID-19 Deaths [Internet]. 2021. Accessed January 14, 2024. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/racial-ethnicdisparities/disparities-deaths.html
- 23.Phelan J.C., Link B.G., Tehranifar P. Social conditions as fundamental causes of health inequalities: theory, evidence, and policy implications. J Health Soc Behav. 2010;51(Suppl):S28–S40. doi: 10.1177/0022146510383498. [DOI] [PubMed] [Google Scholar]
- 24.Vyas D.A., Eisenstein L.G., Jones D.S. Hidden in plain sight - reconsidering the use of race correction in clinical algorithms. N Engl J Med. 2020;383:874–882. doi: 10.1056/NEJMms2004740. [DOI] [PubMed] [Google Scholar]
- 25.Lederer D.J., Benn E.K., Barr R.G., Wilt J.S., Reilly G., Sonett J.R., et al. Racial differences in waiting list outcomes in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:450–454. doi: 10.1164/rccm.200708-1260OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emanuel E.J., Persad G., Upshur R., Thome B., Parker M., Glickman A., et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382:2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 27.Biddison E.L.D., Gwon H.S., Schoch-Spana M., Regenberg A.C., Juliano C., Faden R.R., et al. Scarce resource allocation during disasters: a mixed-method community engagement study. Chest. 2018;153:187–195. doi: 10.1016/j.chest.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Truog R.D., Mitchell C., Daley G.Q. The toughest triage - allocating ventilators in a pandemic. N Engl J Med. 2020;382:1973–1975. doi: 10.1056/NEJMp2005689. [DOI] [PubMed] [Google Scholar]
- 29.Brown M.J., Goodwin J. Allocating medical resources in the time of Covid-19. N Engl J Med. 2020;382:e79. doi: 10.1056/NEJMc2009666. [DOI] [PMC free article] [PubMed] [Google Scholar]