Figure. 28-Day Risk of Adverse Events Following Vaccination With an XBB.1.5-Containing Vaccine.
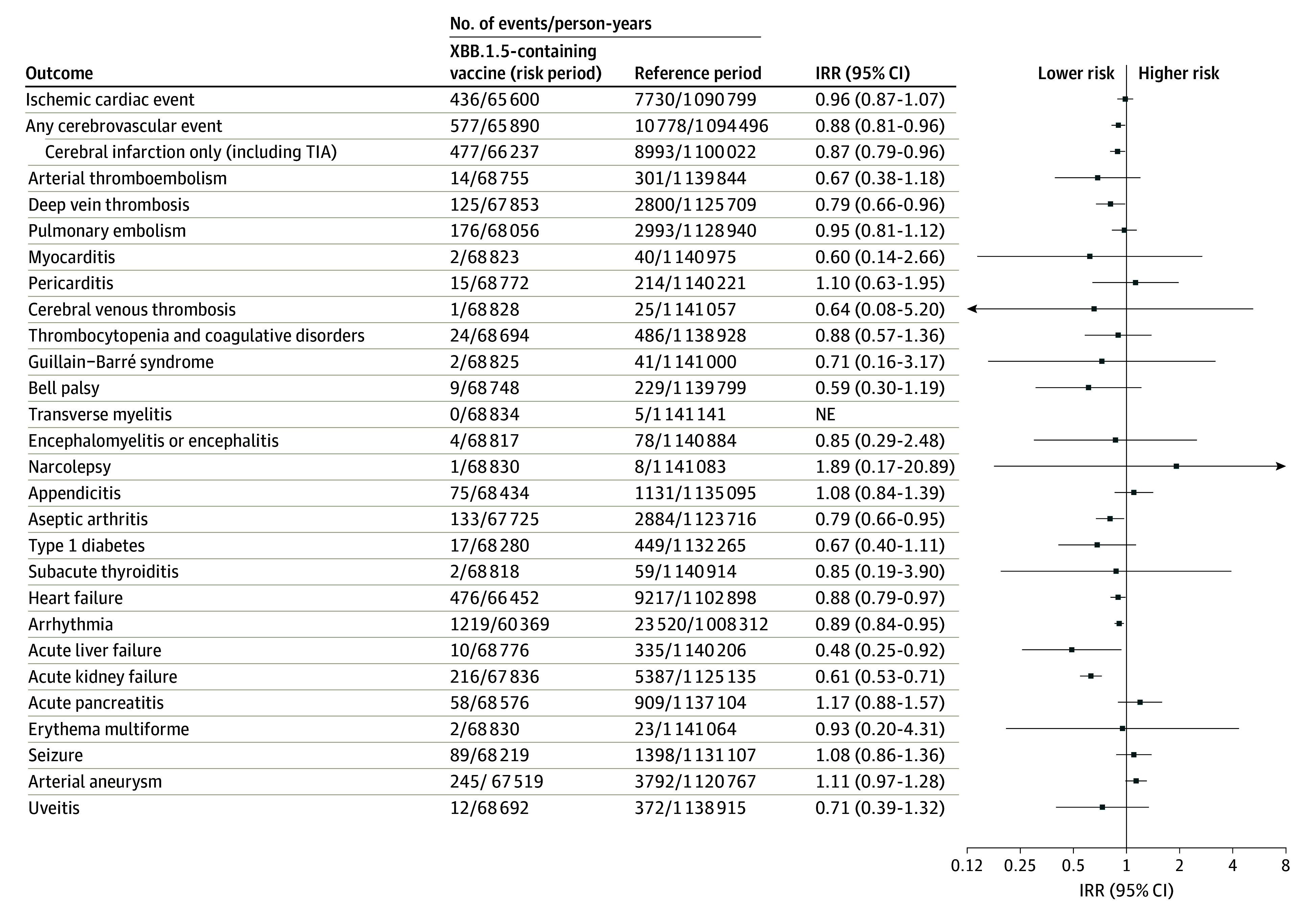
Shown are the 28-day risk period rates of the 28 included adverse events of special interest to COVID-19 vaccines following XBB.1.5-containing mRNA vaccine immunization as a fifth dose compared with reference period rates in Danish people aged 65 years and older from October 1, 2023, to January 8, 2024. The 28-day risk period outcome rates following fifth dose vaccination with an XBB.1.5-containing mRNA vaccine was compared with reference period rates from day 43 after the fourth or fifth dose and onward. Individuals could contribute with person-time during both the 28-day risk period and the 2 reference periods while the number of events and person-time from the 2 reference periods were aggregated. Each outcome was studied separately, which is why there may be slight differences in the denominators due to different exclusions. The arrows indicate that the 95% CI exceeds the upper or lower limits on the x-axis. IRR indicates incidence rate ratio; NE, not estimable; and TIA, transient ischemic attack.
