Figure 1. K+ channel blocker rescue of VAMP2 mutation-induced exocytosis defects in vitro.
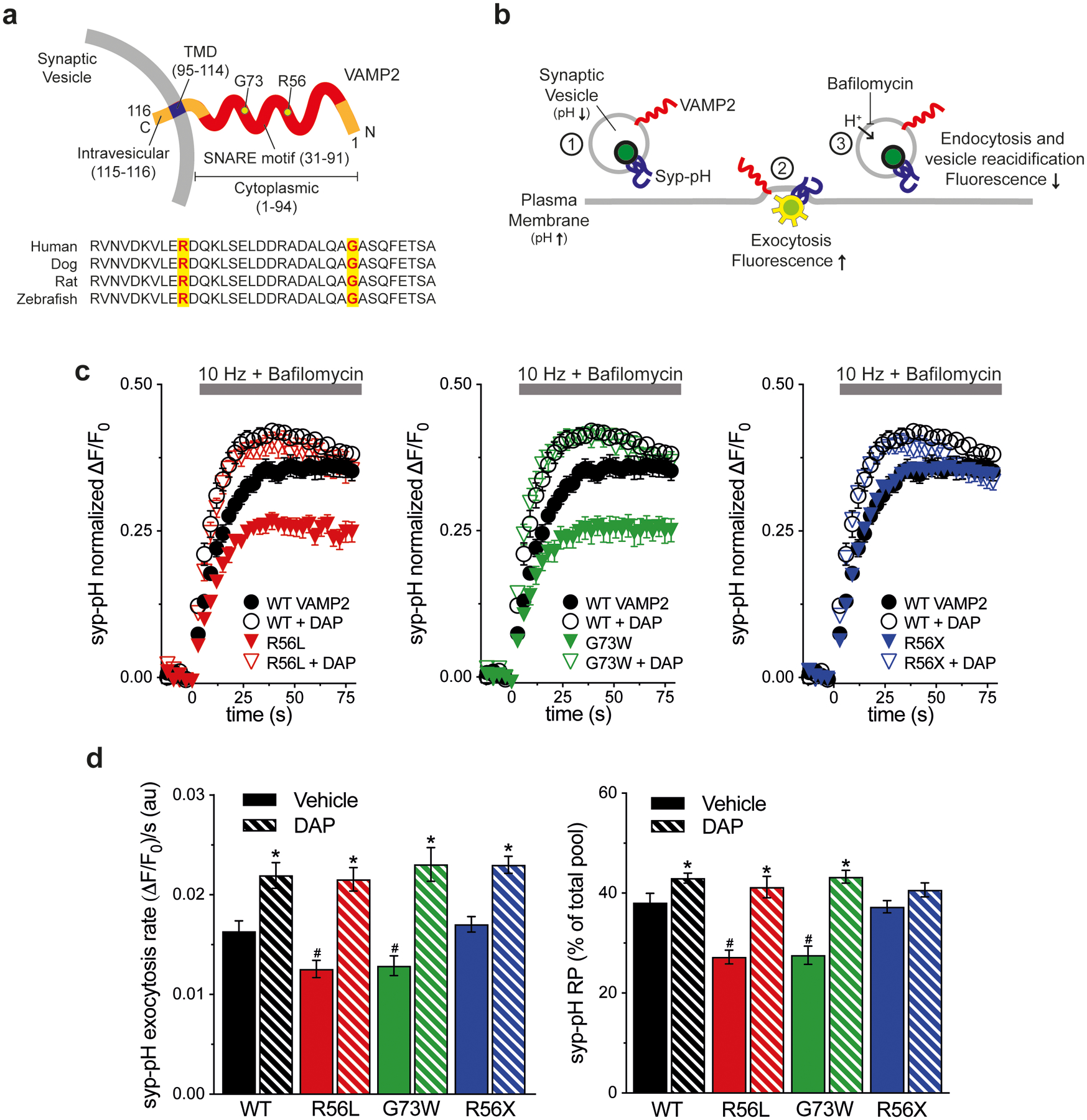
(a) Schematic of human VAMP2 depicts the C-terminal SV transmembrane domain (TMD), cytoplasmic SNARE motif, and variant amino acids. Alignment shows sequence conservation of SNARE across species. (b) Schematic of live cell imaging of SV recycling. Syp-pH fluorescence is quenched at low SV pH (1). Electrical stimulation to elicit exocytosis relieves fluorescence quenching upon exposure of the luminal syp-pH to higher external pH (2). After stimulation, syp-pH fluorescence decreases upon endocytosis and reacidification of SVs by the vacuolar H+-ATPase (3). The vacuolar H+-ATPase inhibitor bafilomycin in the external media blocks reacidification of SVs that have taken up the drug, eliminating fluorescence changes due to endocytosis (3). (c) Time course of exocytosis in response to 10 Hz stimulation in bafilomycin, in neurons co-transfected with the indicated VAMP2-mOr2 constructs, with vehicle or DAP. (d) Quantification of the rate and extent of exocytosis from the recycling SV pool (RP), as a percent of the total pool. Arg56Leu (p.R56L) and Gly73Trp (p.G73W) variants decrease exocytosis rate and extent compared to WT (#P<0.05 for each). Arg56X (p.R56X) truncation is similar to WT. DAP increases the extent of exocytosis with WT, p.R56L, and p.G73 variants, compared to control (*P<0.05). Exocytosis rate is increased with all VAMP2 constructs (*P<0.01 each). Data are means ± SEM of the change in fluorescence (ΔF) normalized to initial fluorescence (F0) over at least 23 boutons per coverslip from 9–12 coverslips from at least three independent cultures. Significance determined by t-tests.
