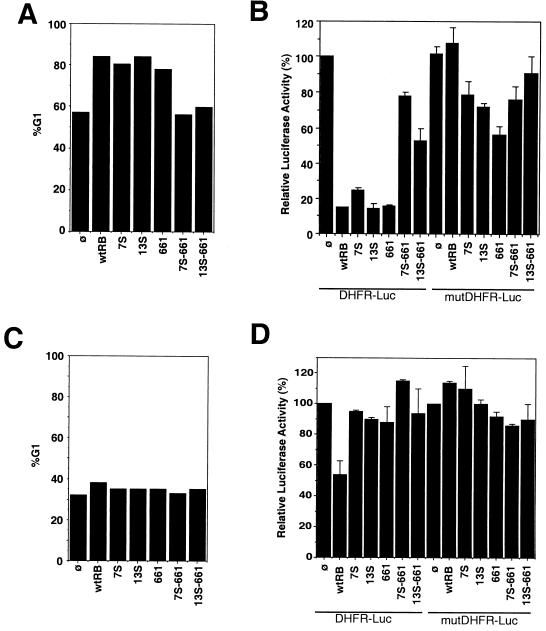
FIG. 4.
Abilities of RB mutants to induce G1 arrest. (A) Induction of an increase in the G1 population of Saos-2 cells. Saos-2 cells were cotransfected with CMV–β-galactosidase, CD20, the DHFR-Luc reporter, or the mutDHFR-Luc reporter and either the vector, wild-type RB (wtRB), 7S, 13S, 661, 7S-661, or 13S-661 (as described in Materials and Methods). One-half of each transfection mixture was used for cell cycle analysis, and the other half was used for a luciferase assay (B). Transfected cells were stained for CD20 and DNA (see Materials and Methods). The gated CD20-positive cells were analyzed by FACS analysis for DNA content. Results of a representative experiment are shown. Similar results were obtained from three additional independent experiments. %G1, percent increase in number of cells in G1. (B) Repression of the DHFR promoter in Saos-2 cells. One-half of the transfected Saos-2 cells in panel A were analyzed for luciferase activity, which was normalized to cotransfected β-galactosidase activity to correct for transfection efficiency. The normalized luciferase activities are represented as percentages of the luciferase activity of vector-cotransfected cells (ø), which is considered 100%. Results shown are the means and standard deviations of results from four independent experiments. (C) Inability of RB to induce an increase in the G1 population of C33A cells. C33A cells were cotransfected with CMV–β-galactosidase, CD20, the DHFR-Luc reporter, or the mutDHFR-Luc reporter and either the vector, RB, 7S, 13S, 661, 7S-661, or 13S-661 as described for panel A. Transfected cells were stained for CD20 and DNA, and the percentages of cells in G1 were determined as described in Materials and Methods. (D) Repression of the DHFR promoter in C33A cells. C33A cells were analyzed as described for panel B. Normalized luciferase activities are represented as percentages of the luciferase activity of vector-cotransfected cells (ø), which is considered 100%. Results shown are the means and standard deviations of results from four independent experiments.