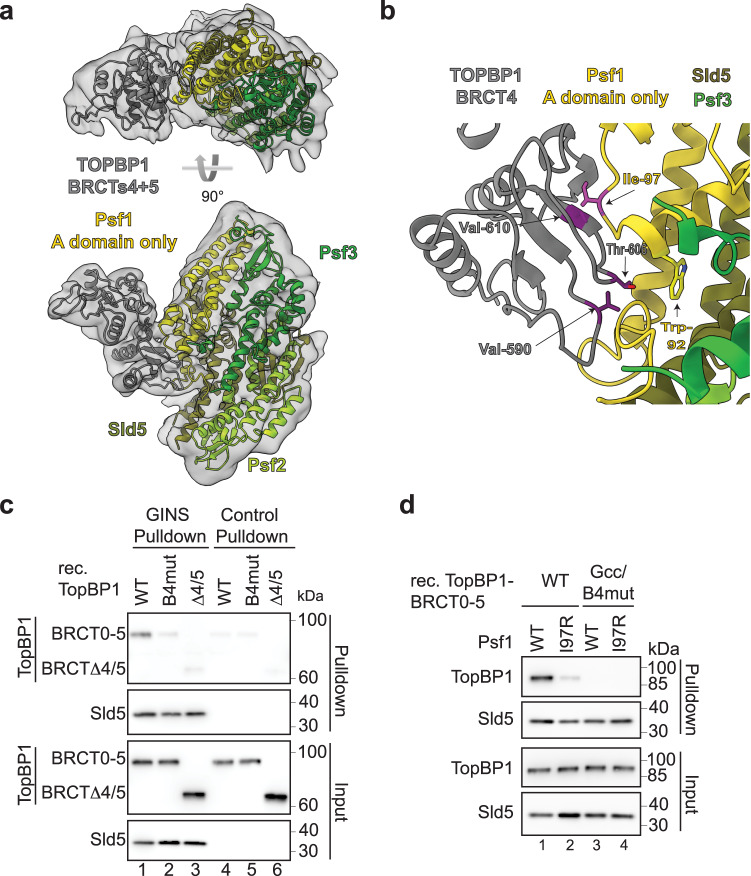
Fig. 2. Protein structure of the GINS-TopBP1-BRCT4/5 complex.
a Structural model of the GINS-TopBP1-BRCT4/5 complex. Crystal structures of the GINS subunits (PDB:2E9X) (shades of green, yellow), and the central BRCT4/5 domain of TopBP1 (PDB:3UEN) (grey) were docked into the cryo-EM volume shown as transparent volume. b Zoom-in of the BRCT4-Psf1 interface. The residues in stick representation appear crucial for the interface. Mutations to break the interaction are coloured pink (Psf1-I97R) and purple (TopBP1-B4mut; compare Fig. 1a(ii)). c Pulldown of the indicated recombinant TopBP1-BRCT0-5-strep versions (see Fig. 1a(ii)) by Flag-beads-immobilised recombinant GINS or Flag peptide-coupled control beads. Analysis was done by immunoblotting. The experiment was done more than three times with similar results. d Pulldown of the indicated recombinant TopBP1-BRCT0-5-WT-strep using immobilised GINS-WT or GINS carrying a Ile97 to arginine mutation in Psf1.TopBP1-Gcc/B4mut-strep (Figs. 1a(ii) and 3a) was used as a non-GINS binding control. The experiment was done twice with similar results. Source data are provided as a Source data file.