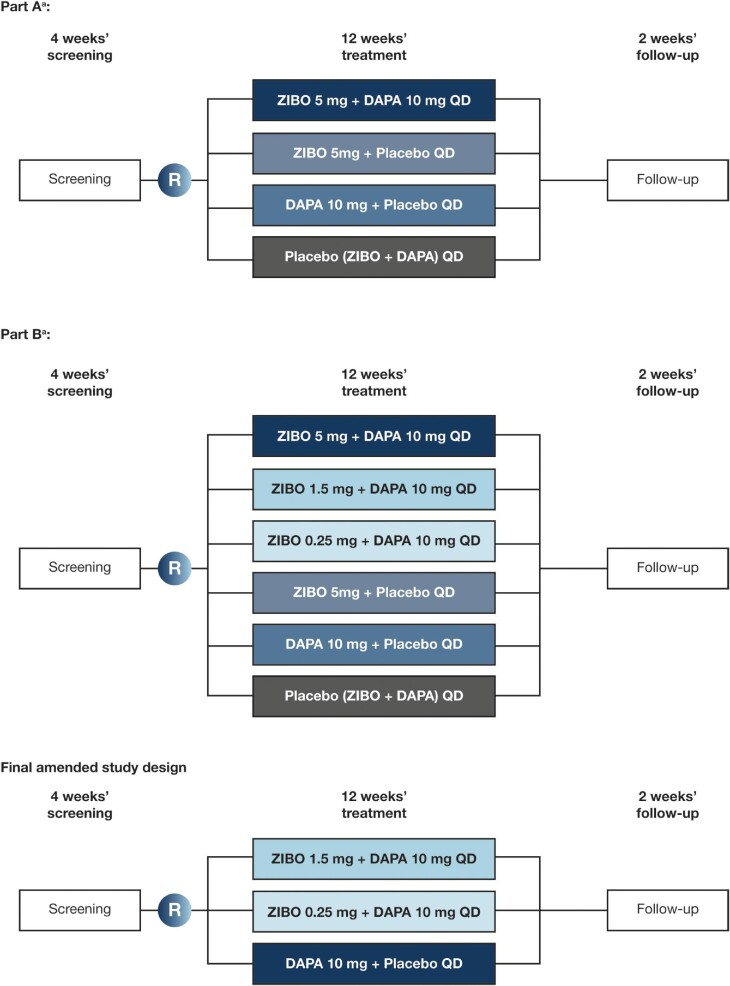
Figure 2:
ZENITH-CKD study design. aFollowing an ad hoc safety review, a protocol amendment was implemented on 5 April 2022. Owing to the rate of fluid-retention events in the zibotentan 5 mg QD and the zibotentan 5 mg/dapagliflozin 10 mg QD groups, randomization to these groups was closed. DAPA, dapagliflozin; R, randomization; ZIBO, zibotentan.