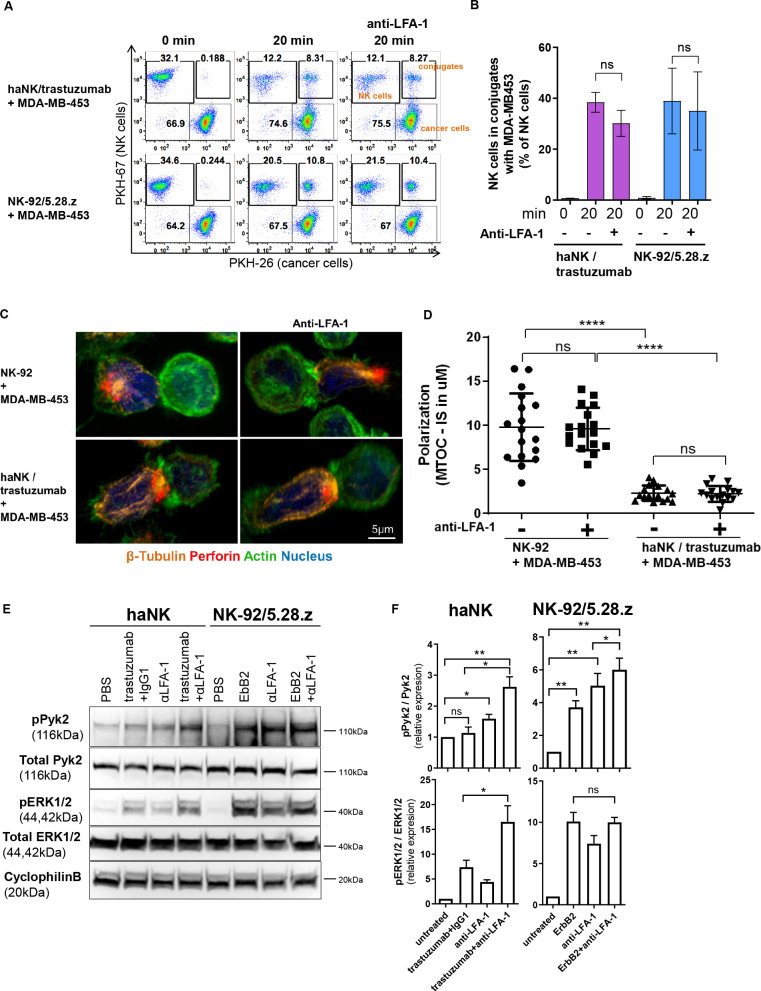
Figure 7.
Chimeric antigen receptor activation can substitute for LFA-1 signaling by activating the Pyk2 pathway. (A) PKH-67-labeled haNK cells combined with trastuzumab or PKH-67-labeled NK-92/5.28.z cells were blocked with anti-LFA-1 antibody, and incubated with PKH-26-labeled MDA-MB-453 target cells for 20 min at 37°C as indicated. Control samples at 0 min were used to exclude unspecific binding or cross-staining. Cells were fixed and analyzed for conjugate formation by flow cytometry. (B) Quantification of conjugate formation. Pooled data from three independent experiments performed in triplicate are shown. Data represent mean values±SEM. (C) Confocal microscopy of NK-92 cells, and haNK cells combined with trastuzumab, incubated with MDA-MB-453 target cells. NK cells were blocked with anti-LFA-1 antibody prior to exposure to cancer cells as indicated. Representative images are shown. (D) Microtubule-organizing center (MTOC) polarization toward the immunological synapse (IS) was quantified from three independent experiments. Mean values±SEM are shown. (E) haNK or NK-92/5.28.z cells were stimulated with plate-bound trastuzumab, anti-LFA-1 antibody, ErbB2, or isotype control antibody as indicated. Cell lysates were prepared and analyzed by immunoblotting for phospho-Pyk2 (pPyk2; Tyr402) and phospho-ERK1/2 (pERK1/2; Thr202/Tyr204). As controls, total Pyk2 and ERK1/2 levels were analyzed. Cyclophilin B was used as a loading control. (F) Quantification of Pyk2 and ERK1/2 phosphorylation normalized to total protein levels. Data were pooled from three independent experiments. Mean values±SEM are shown. haNK, high-affinity FcγRIIIa-modified NK-92 cells; NK, natural killer; PBS, phosphate-buffered saline.