Figure 1.
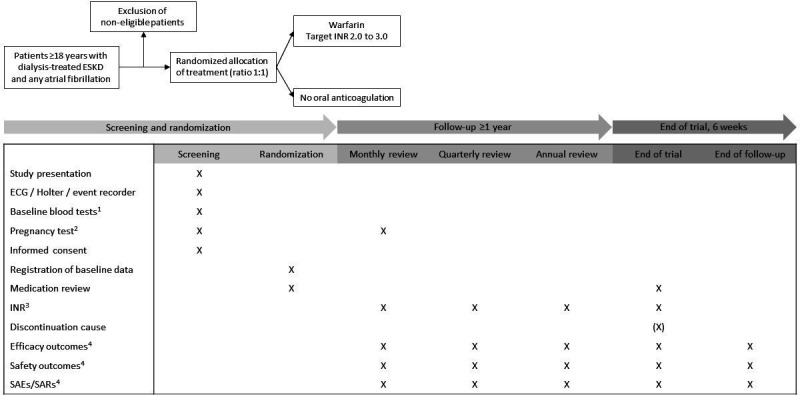
Study design. Eligibility is assessed in accordance with the inclusion and exclusion criteria. 1Plasma-haemoglobin, platelet count, albumin, phosphate, ionised calcium, parathyroid hormone, C reactive protein, urea nitrogen and creatinine. 2Required in all women of childbearing potential at inclusion and monthly throughout the trial. 3Last recorded INR 4SAEs/SARs will be recorded continuously with reporting of SAEs/SARs to the study sponsor within 24 hours of identification for assessment. Efficacy and safety outcomes will be registered every month in an electronic database. ESKD, end-stage kidney disease; INR, international normalised ratio; SAE, serious adverse event; SAR, serious adverse reaction.
