Abstract
Exosomes, extracellular vesicles secreted by cells, have garnered significant attention in recent years for their remarkable therapeutic potential. These nanoscale carriers can be harnessed for the targeted delivery of therapeutic agents, such as pharmaceuticals, proteins, and nucleic acids, across biological barriers. This versatile attribute of exosomes is a promising modality for precision medicine applications, notably in the realm of cancer therapy. However, despite their substantial therapeutic potential, exosomes still confront challenges tied to standardization and scalability that impede their practice in clinical applications. Moreover, heterogeneity in isolation methodologies and limited cargo loading mechanisms pose obstacles to ensuring consistent outcomes, thereby constraining their therapeutic utility. In contrast, exosomes exhibit a distinct advantage in cancer diagnosis, as they harbor specific signatures reflective of the tumor’s genetic and proteomic profile. This characteristic endows them with the potential to serve as valuable liquid biopsies for non-invasive and real-time monitoring, making possible early cancer detection for the development of personalized treatment strategies. In this review, we provide an extensive evaluation of the advancements in exosome research, critically examining their advantages and limitations in the context of cancer therapy and early diagnosis. Furthermore, we present a curated overview of the most recent technological innovations utilizing exosomes, with a focus on enhancing the efficacy of early cancer detection.
Keywords: exosome, cancer, diagnosis, therapy, translational research
1 Introduction
Cancer, an intricate and pervasive disease, presents a formidable global threat to human life. With a reach that spans across age, gender, and diverse backgrounds, cancer strikes fear into millions of the population (Thill et al., 2023; Wolf et al., 2023). Moreover, cancer places a substantial economic burden on healthcare systems and societies worldwide due to escalating costs associated with treatment and supportive care (Amini et al., 2022; Munshi et al., 2022). What makes cancer particularly challenging is its capacity to infiltrate any organ or tissue, from the lungs to the skin, and from the brain to the bones (Hayashi et al., 2023; Li and Zheng, 2023). Often, its stealthy progression remains concealed until it develops an advanced stage (i.e., usually hard to treat), rendering early detection and treatment a critical challenge (Aleksandrowicz et al., 2023; Wang W T et al., 2023).
Curing cancer is an exceptionally tough endeavor, largely due to the obstacle known as metastasis (Hu Z et al., 2023; Zhou et al., 2023). Metastasis entails cancer cells breaking away from the primary tumor, traversing the bloodstream or lymphatic system, and establishing secondary tumors in distant body parts. This ability to spread and infiltrate new organs or tissues renders cancer highly elusive and resistant to conventional treatment approaches (Hayashi et al., 2023; Li and Zheng, 2023). By the time metastasis is detected, cancer is often at an advanced stage, making complete eradication considerably more challenging. Each secondary tumor presents unique challenges, demanding specific approaches, and the intricate complexity of metastatic disease necessitates a multifaceted treatment strategy (Feng et al., 2023; Gao et al., 2023). Furthermore, cancer cells can evolve, developing resistance to previously effective therapies (Gera et al., 2023; Mahmoud et al., 2023). Therefore, early detection of cancer before its metastasis is critical for cancer treatment and enhances the survival of cancer patients. However, tackling metastasis remains one of the most formidable hurdles on the path to finding a cure for cancer (Wang et al., 2023a; Holladay et al., 2023).
Traditional cancer diagnostic techniques, while historically invaluable, grapple with significant limitations (Xiao, 2015). Many of these methods require invasive procedures like surgeries to obtain tissue samples for analysis, which can be painful and carry risks, making them unsuitable for the patients (Zhang et al., 2013). Additionally, these conventional diagnostic approaches can incur prohibitively high costs, burdening both healthcare systems and patients, particularly when multiple tests are necessary to confirm a diagnosis or monitor treatment progress. Furthermore, their sensitivity, especially in detecting early-stage cancers, can be limited, resulting in smaller tumors or cancerous cells missing until they reach more advanced and less treatable stages (Bassani et al., 2023; Kawada et al., 2023; Tan and Hosein, 2023; Zhang Y et al., 2023). To overcome these limitations, there has been a growing endeavor on developing non-invasive, cost-effective, and highly sensitive diagnostic methods, such as liquid biopsies and advanced imaging technologies (Young et al., 2023). These innovations hold the potential to revolutionize cancer diagnosis by offering earlier detection, reduced invasiveness, and more precise monitoring, ultimately enhancing our ability to combat this devastating disease.
Exosomes have emerged as a burgeoning field of research with vast potential in the realm of cancer detection (Hyun et al., 2018; Xia et al., 2020). These nanoscale particles, once dismissed as cellular debris, are now recognized for their pivotal role in intercellular communication and their capacity to transport molecular cargo, including nucleic acids and proteins (Zhang et al., 2016; Maia et al., 2018). In the context of cancer development, exosomes assume a critical role by ferrying tumor-specific molecules that can serve as biomarkers for early detection (Regev-Rudzki et al., 2013). Their presence in bodily fluids like blood and urine renders them a highly attractive source for non-invasive and easily accessible diagnostic tests (Agnoletto et al., 2023; Wang, et al., 2023b). Researchers are increasingly harnessing the exosomes to detect cancer at its earliest stages, especially before the metastasis when treatment interventions are most effective (Lin et al., 2021; Yu et al., 2021). Furthermore, the diverse array of information conveyed within exosomes offers a plethora of potential markers, enabling not only the detection of cancer but also the identification of specific cancer types and their distinctive molecular characteristics (Sharma and Salomon, 2020). This approach holds significant promise for developing specific treatment strategies for individual patients, thereby improving treatment outcomes and reducing the burden of unnecessary treatments (Kumata et al., 2018; Luo et al., 2018; Smith and Lam, 2018).
In conclusion, the investigation of exosomes as a method for cancer detection marks a promising and innovative Frontier in the field of oncology research (Li G et al., 2020; Zhang W et al., 2021). It holds the tremendous potential to reform the field of early diagnosis, monitoring, and treatment decisions on cancers. As innovative methods continue to emerge in this burgeoning field, exosomes have the potential to become a pivotal element in combatting cancer. They offer patients more efficient and minimally invasive diagnostic alternatives, potentially enhancing treatment efficacy, prolonging survival, and significantly improving the quality of life for those affected by cancer.
2 Generation of exosome and its function
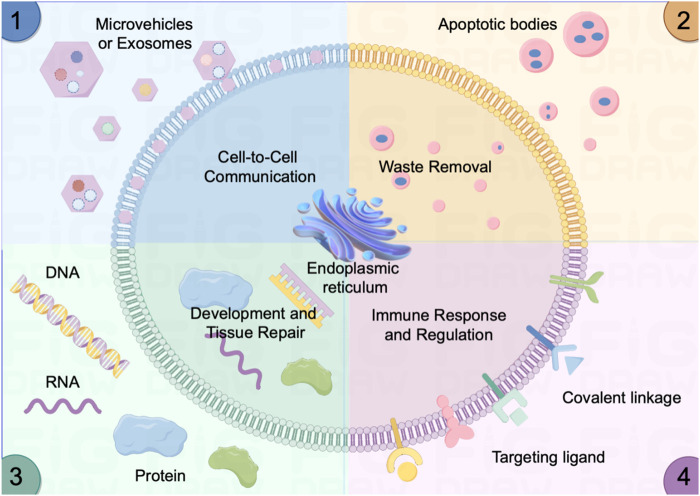
Exosomes play a crucial role in cellular quality control, ensuring the maintenance of cellular health. This assures their potential therapeutic applications, especially in conditions marked by impaired waste removal processes (Kakiuchi et al., 2023; Zhao J et al., 2023). The primary functions of exosomes are sketched in Figure 1.
FIGURE 1.
Sketch of the activity and functions of the micro vehicles/exosome in vivo. (1) Cell-Cell Communication: Mediate intercellular communication by transporting essential molecules, fostering coordination in physiological processes. (2) Efficient Waste Removal: Serve as carriers for cellular waste removal, contributing to cellular cleanliness (e.g., apoptotic bodies) and overall tissue health. (3) Developmental Regulation and Tissue Repair: Regulate developmental processes and tissue repair by delivering signaling molecules (e.g., DNA, micro RNAs, and proteins) that influence cellular activities. (4) Immune Response Modulation: Actively modulate immune responses by conveying signals via targeting ligand and covalent linkage between immune cells and somatic cells, contributing to inflammation regulation and immune system balance.
2.1 Cell-to-cell communication
Exosomes play a fundamental role in the intricate network of cell-to-cell communication within biological systems. These nanoscale vesicles serve as essential messengers between cells, functioning as conduits for the intercellular exchange of diverse molecular cargo, including proteins, nucleic acids (both DNA and RNA), and lipids (Luo et al., 2023; Shang et al., 2023). When a cell secretes exosomes, they can be internalized by neighboring or distant recipient cells, thereby facilitating the transfer of vital information (Altintas and Saylan, 2023). This molecular exchange enables cells to coordinate and regulate a wide array of cellular activities and responses. For instance, immune cells utilize exosomes to convey signals that orchestrate immune responses, while cancer cells exploit exosome communication to promote tumor growth and metastasis (De La Pena et al., 2009; Kang et al., 2023).
2.2 Waste removal
In the network of cellular metabolite maintenance, exosomes serve as indispensable tools for waste removal and cellular quality control mechanisms (Lu et al., 2023). Cells harness the remarkable capacity of exosomes to encapsulate and transport a variety of unwanted or degraded cellular components, such as misfolded proteins, broken organelles, and waste of metabolites, to facilitate their efficient elimination from the cell (Gudbergsson and Johnsen, 2019; Schubert, 2020). This waste disposal process not only ensures the preservation of cellular integrity and functionality but also contributes to the overall health and homeostasis of multicellular organisms. Moreover, the role of exosomes in waste removal extends beyond the individual cell, as they can be released into extracellular environments, such as bodily fluids, where they can aid in systemic waste clearance and potentially play a role in intercellular signaling (Nik Mohamed Kamal and Shahidan, 2021; Lai et al., 2023).
2.3 Development and tissue repair
Exosomes also serve as pivotal mediators in critical biological processes, including tissue regeneration and development (Li et al., 2015; Pan et al., 2023). They orchestrate the process of growth and repair in tissues by facilitating the transfer of growth factors and an array of signaling molecules to target cells (Wang and Yang, 2023; Zhao X et al., 2023). In the context of tissue regeneration, exosomes act as specialized messengers, guiding and stimulating cellular responses necessary for the healing and rebuilding of damaged or injured tissues. Notably, in the nervous system, exosomes play an indispensable role in neuronal communication (i.e., synapse) and maintenance (Liu et al., 2022). They serve as nanocarriers, shuttling neurotransmitters, with a myriad of signaling molecules between neurons and other supporting cells within the brain. This intercellular exchange not only underpins fundamental processes in neural development but also contributes to the fine-tuning of neural circuits, highlighting the profound impact of exosomes on neurological function and giving potential therapeutic applications in neurodegenerative diseases and neurological disorders (Hu T et al., 2023; Liu et al., 2023; Zhong et al., 2023).
2.4 Immune response regulation
Exosomes assume a crucial role in orchestrating immune responses, wielding their influence in the intricate domain of immune system function (Schwarzenbach and Gahan, 2021). Immune cells harness the power of exosomes to disseminate signaling molecules that serve as directives for the regulation of immune responses, effectively coordinating the complex interplay of immune mechanisms (Greening et al., 2015; Chen et al., 2017; Li et al., 2019). Moreover, exosomes act as couriers of information, transferring crucial insights about pathogens or abnormalities to other immune cells, thereby enabling swift and coordinated countermeasures against infections or aberrant cell behavior (Kaminski et al., 2019; Lema and Burlingham, 2019). In the realm of disease, exosomes take on added significance as potential diagnostic tools. For instance, cancer cells exploit exosomes as conveyors of disease-specific cargo, including tumor-specific proteins and genetic material which further modulate the immune response (Anel et al., 2019; Shen et al., 2019).
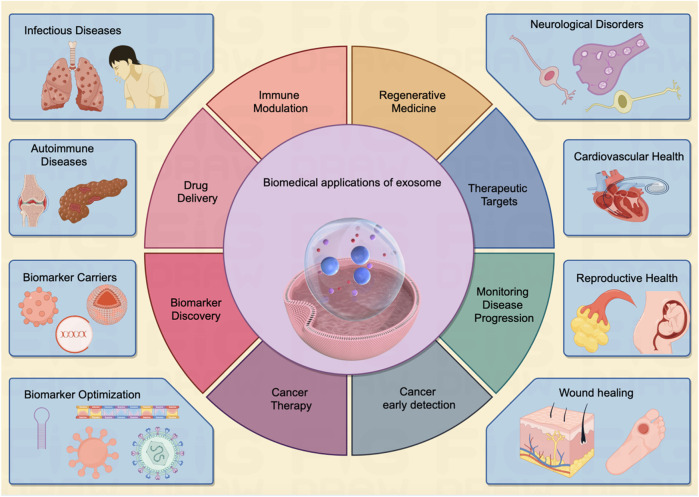
Of note, exosomes have garnered significant attention in the field of cancer research, as they are believed to play a critical role in cancer progression, metastasis, and drug resistance (Lema and Burlingham, 2019; Shen et al., 2019). Researchers are exploring the potential of exosomes as diagnostic markers and therapeutic targets in various diseases, including cancer, neurodegenerative disorders, and autoimmune conditions (Figure 2). Their ability to carry specific molecular cargo makes them a promising avenue for understanding disease mechanisms and developing novel medical treatments (Table 1).
FIGURE 2.
The exosome exerts multi-faceted biomedical potentials for human disease. Exosomes demonstrate a vast array of biomedical applications pertinent to human diseases affecting various organs throughout the body. These entities serve as pivotal biomarkers in diagnostic processes and hold considerable potential across multiple domains, including targeted drug delivery, immunomodulation, and tissue regeneration. As multifunctional agents, they are increasingly acknowledged as instrumental in driving forward the Frontier of innovative biomedical research, heralding a notable shift in the paradigms of disease detection and therapeutic strategies, as illustrated in the center panel. The inherent attributes of exosomes establish them as an emergent and significant field within biomedical research, poised to radically reshape methodologies in disease diagnosis, therapeutic interventions, and the broader spectrum of healthcare practices.
TABLE 1.
Contents in the exosomes collected for immune response in recent studies.
| Sources | Contents | Effect | References |
|---|---|---|---|
| Alveolar epithelial type II cells | Viral particles | Help SARS-CoV for immune system evasion | Qian et al. (2013); Mason (2020) |
| CD4+ T cells | Proteins of HIV | Inhibit and induce the apoptosis of CD4+ T cells | Ali et al. (2010), Lenassi et al. (2010), de Carvalho et al. (2014) |
| Dendritic cells | MHC-I and MHC-II | Associated with T-cell-dependent anti-tumor response | Hsu et al. (2003) |
| Glioma | DNA | Cross the intact blood-brain barrier and present in peripheral blood | García-Romero et al. (2017) |
| HIV-infected cells | Viral nucleic acids of HIV | Deliver viral nucleic acids to the health cells | He et al. (2010), Narayanan et al. (2013) |
| Human breast milk | Oligosaccharides | Decrease the inflammation in the gut | Okoye et al. (2014) |
| Intestinal epithelial cells | MHC-II and Fas ligand | Associated with the apoptosis of dendritic cells | Takenaka and Quintana (2017) |
| Intestinal epithelial cells | MHC-II and Fas ligand | Associated with Microglial activation, decline of synaptic stability | Hasegawa and Matsumoto (2018) |
| Intestinal epithelial cells | Integrin αvβ6 | Upregulate the TGF-β in dendritic cells | Hasegawa and Matsumoto (2018) |
| Lung adenocarcinoma | dsDNA | Double-stranded DNA in exosomes of malignant pleural effusions, biomarkers for lung adenocarcinoma | Loscocco et al. (2019) |
| Lymphocytes | miR-142-3p, miR-142-5p, and miR-155 | Associated with the apoptosis of the beta cells in pancreas | Palmisano et al. (2012) |
| Macrophages | miRNA-88 and miRNA-99 | activate the tumor necrosis factor-alpha in the immune response to HIV infection | Bernard et al. (2014) |
| Megakaryocytes and platelets | C-X-C chemokine receptor type 4 (CXCR4) | Increase the susceptibility of CXCR4-null cells respond to HIV infection | Rozmyslowicz et al. (2003) |
| Melanoma | dsDNA | Double-stranded DNA (dsDNA) as a potential biomarker for melanoma detection | Thakur et al. (2014) |
| NSC-34 motor neurons | miR-124 | Induce senescence in microglia cells and reduce phagocytic abilities | Zhao et al. (2019) |
| Osteosarcoma | Repetitive element DNAs | Potential biomarkers for osteosarcoma | Cambier et al. (2021) |
| Ovarian cancer | mtDNA | mtDNA in exosomes, biomarkers for ovarian cancer | Keseru et al. (2019) |
| Regulatory T cells | Let-7d | Suppress the proliferation of Th1 cell | Kurakevich et al. (2013) |
| Serum | miR-424-5p | Inhibit granulosa cell proliferation and induce cell senescence for the patients with polycystic ovary syndrome | Bozdag et al. (2016) |
3 The biomedical application of exosome
Exosomes have gained immense significance in the field of biomedicine due to their diverse and versatile applications (Greening et al., 2015). These exosomes carry a variant of molecular cargo, making them valuable tools for numerous biomedical applications (Whiteside, 2017). The key biomedical applications of exosomes are indicated in Figure 2.
3.1 Regenerative medicine
Exosomes derived from stem cells contain growth factors and signaling molecules that can stimulate cell proliferation and tissue healing. Exosome-based therapies are being explored for conditions like heart disease and tissue injuries (Zhang and Cheng, 2023). It can be used in more multiple-system disease treatments such as follows:
Neurological disorders: Exosomes are involved in intercellular communication in the nervous system. They may play a role in the pathogenesis of neurodegenerative diseases such as Alzheimer’s and Parkinson’s. Studying exosomes in these contexts can provide insights into disease mechanisms and potential therapeutic interventions (Xiao et al., 2021). Recent studies show that exosomes from neural progenitor cells promote neuronal differentiation and neurogenesis via miR-21a (Ma et al., 2019). Similarly, exosomes from human induced pluripotent stem cell (hiPSC)-derived neurons enhance proliferation in human primary neural cultures in vitro. Injection of exosomes from rodent primary neural cultures into P4 mouse brains also increases neurogenesis in the dentate gyrus of the hippocampus (Sharma et al., 2019).
Cardiovascular health: Exosomes are being explored as diagnostic and therapeutic tools in cardiovascular diseases. They can carry molecules associated with heart conditions and may be used to monitor and treat heart diseases (Li N et al., 2023). Wang et al. report that exosomes from induced pluripotent stem cells (iPSCs) transfer cardioprotective miRNAs, including miR-21 and miR-210, to cardiomyocytes. Elevated miRNA levels in iPSC-exosomes contribute to protection against oxidative stress in H9C2 cells by inhibiting caspase 3/7 activation. These anti-apoptotic effects were validated in a mouse model of acute myocardial ischemia/reperfusion (Zhang et al., 2015).
Reproductive health: Exosomes play roles in reproductive biology, including sperm function and embryo development. They may have applications in fertility treatments and the study of reproductive disorders (Ranjbaran et al., 2019; Pu et al., 2022). Zhao et al. explored the impact of exosomal miR-323-3p from adipose mesenchymal stem cells (AMSCs) on cumulus cells (CCs) of polycystic ovary syndrome (PCOS) patients, finding that it inhibited apoptosis by targeting programmed cell death protein 4 (PDCD4) and alleviated PCOS (Zhao et al., 2019). Another study revealed that exosomes from the serum of PCOS patients significantly stimulated the migration and invasion of endometrial cancer cell lines. The study identified miR-27a-5p as highly induced in serum exosomes from PCOS patients and demonstrated its direct targeting of the tumor suppressor gene SMAD4 in the TGF-β signaling pathway (Che et al., 2020).
Wound healing: Exosomes have shown great promise in wound healing due to their ability to carry and deliver bioactive molecules, such as growth factors and microRNAs, to the damaged tissue. These exosomes can promote tissue regeneration, reduce inflammation, and accelerate the overall wound-healing process (Kudinov et al., 2021; Geng et al., 2022). A study engineered MSCs to produce exosomes enriched with long non-coding RNA H19, regulating the PI3K/AKT pathway and suppressing inflammation and apoptosis in a diabetic foot ulcer mouse model (Li B et al., 2020). Additionally, nanoparticles loaded into exosomes, specifically BMSCs-exosomes with magnetic Fe3O4 nanoparticles and increased exosome miR-1260a, were found to boost angiogenesis (Wu et al., 2021).
3.2 Immune modulation
Exosomes derived from immune cells play a pivotal role in orchestrating immune responses within the body (Gangadaran et al., 2022b). These tiny vesicles serve as potent messengers of intercellular communication, carrying a cargo of bioactive molecules such as cytokines, chemokines, and antigens (Trams et al., 1981). When immune cells encounter pathogens or foreign invaders, they release exosomes that can activate or suppress immune reactions, depending on the context (Table 1). For instance, dendritic cell-derived exosomes can present antigens to T cells, initiating adaptive immune responses against infections or cancer (Shenoda and Ajit, 2016). Conversely, regulatory T cell-derived exosomes can exert immunosuppressive effects, modulating excessive inflammation and autoimmunity (Arenaccio et al., 2014). Numerous studies highlight the role of CD8+ T cell-derived exosomes in facilitating communication between immune cells and tumor cells, regulating tumor development. Fully activated CD8+ CTLs release exosomes that boost the activation of low-affinity CD8+ T cells, contributing to the tumor-killing process (Li et al., 2017; Wu S W et al., 2019). Understanding the intricate role of immune cell-derived exosomes is crucial for advancing our knowledge of immune regulation and developing novel therapeutic strategies for immune-related disorders.
3.3 Drug delivery
Exosomes also have emerged as promising natural nanocarriers for drug delivery in recent years (Gangadaran et al., 2022a). First, their natural origin minimizes immunogenicity and toxicity concerns, making them well-tolerated in vivo. Second, exosomes are equipped with cell-targeting proteins and bioactive molecules on their surface, facilitating specific delivery to target cells or tissues (Gangadaran et al., 2022c). Furthermore, their stability in circulation and ability to traverse biological barriers, such as the blood-brain barrier, offer a versatile platform for encapsulating various therapeutic cargoes, including small molecules, nucleic acids, and proteins (Krishnan et al., 2022). Harnessing exosomes as nanocarriers holds great potential to enhance the precision and efficacy of drug delivery in diverse therapeutic applications.
3.4 Biomarker discovery for cancer treatment
Exosome cargo analysis can facilitate the discovery of disease-specific biomarkers. These biomarkers can be used for early disease detection, monitoring disease progression, and assessing treatment responses. Of note, the exosome is promising for the cancer diagnostics (Si et al., 2023). Exosomes derived from cancer cells contain specific biomarkers, such as mutant DNA, proteins, and microRNAs, that can serve as indicators of cancer presence and progression (Ji et al., 2013; Miaomiao et al., 2023). Liquid biopsies based on exosome cargo analysis offer non-invasive methods for early cancer detection and monitoring treatment responses (Feng et al., 2019). Moreover, exosome-based diagnostics and therapeutics represent a promising avenue in cancer therapy (Kalluri and LeBleu, 2020). Researchers are exploring the use of exosomes for targeted drug delivery. By engineering exosomes to carry therapeutic molecules, drugs can be directed specifically to cancer cells, reducing off-target effects and improving treatment efficacy (Barile and Vassalli, 2017; Chen H et al., 2021; Jin et al., 2021). Briefly, enhancing exosome targeting for drug delivery platforms involves selecting specific exosome donors or employing bioengineering techniques. This can be achieved by modifying the exosome surface with homing molecules through ligands, magnetic materials, charge affinity, and pH-responsive motifs (Butreddy et al., 2021). Ultimately, by loading the drug into these modified exosomes, targeted carriers can be created for specific cells or organs, leading to improved clinical treatment outcomes (He et al., 2022). The accumulation of drugs in target sites enhances the efficacy of exosomes while simultaneously reducing off-target effects.
Overall, the biomedical applications of exosomes are vast and continually expanding. Their unique properties as carriers of molecular information, combined with their potential for non-invasive sampling and targeted delivery, make exosomes a promising area of research and development in the quest to better understand, diagnose, and treat a wide range of diseases and medical conditions.
4 The application of exosomes for cancer
Exosomes play a crucial role in cancer progression and metastasis by facilitating the transfer of bioactive molecules between cancer cells and diverse cells in both local and distant microenvironments. This intercellular communication leads to alterations in various cellular and biological functions within the recipient cells (Jiang et al., 2021). Additionally, exosomes serve as indicators of the heterogeneity present in cancer tumors (Al-Nedawi et al., 2008). Distinct subpopulations of cancer cells within a single tumor release exosomes with unique molecular profiles (Maia et al., 2018). Recognizing and understanding this heterogeneity is essential as it can guide treatment decisions and potentially pave the way for personalized therapy strategies.
4.1 Biomarker carriers for early detection of cancers
Exosomes have emerged as a promising Frontier in cancer research and diagnostics, driven by their distinctive cargo (Tai et al., 2018). These microscopic vesicles encapsulate a wealth of information derived from cancer cells, serving as invaluable reservoirs of diverse cancer biomarkers, including genetic mutations, oncogenic proteins, and a spectrum of cancer-specific molecules (Jiang et al., 2021; Liu et al., 2021). The encapsulation of such biomarkers within exosomes is of paramount significance, as they can serve as pivotal indicators of cancer initiation, progression, and response to therapeutic interventions (Table 2).
TABLE 2.
Partial biomarkers collected for cancer detection in recent studies.
| Biomarkers | Cancer type | Signatures | References |
|---|---|---|---|
| Annexins 1 | Glioblastoma | Elevated ANXA1 transcript levels were similarly detected in cases of glioblastoma (GBM) | Mallawaaratchy et al. (2017) |
| Annexins 2 | Breast cancer | Overexpression of Annexin A2 has also been confirmed in various malignancies | Maji et al. (2017) |
| Annexins 6 | Breast cancer | Its role varies depending on the type of cancer, as it can function either as a tumor suppressor or as a promoter of motility | Sakwe et al. (2011) |
| CD151 | Lung cancer | Increased CD151 expression in cancer is associated with an unfavorable prognosis | Niu et al. (2019) |
| CD63 | Melanoma | Reduced expression of CD63 upon cancer progression | Pols and Klumperman (2009) |
| CD81 | Breast cancer | CD81 exhibits increased expression within the stromal region linked to human invasive ductal carcinoma | Luga et al. (2012) |
| CD9 | Prostatic carcinoma | Reduced expression of CD9 in metastatic lesions | Wang et al. (2007) |
| Flotillin2 | Prostatic carcinoma | The upregulated expression of flotillin 2 is associated with cancer progression and prognosis | Wang et al. (2017) |
| GTPase Rab 11 | Colorectal cancer | Overexpression of Rab11 is associated with a prognosis | Chung et al. (2014) |
| GTPase Rab 27 | Lung cancer | Downregulated expression of Rab27 A/B leads to a decrease in the secretion of exosomes from cancer | Li et al. (2014) |
| GTPase Rab 5 | Melanoma | Elevated Rab5 levels can stimulate tumor cell migration | Silva et al. (2016) |
| GTPase Rab 7 | Melanoma | Upregulated Rab7 is an early-acting driver of melanoma that is indicative of patient prognosis | Alonso-Curbelo et al. (2014) |
| Heat shock proteins 60 | Colorectal cancer | Elevated expression of Heat Shock Proteins (HSPs) is indicative of an unfavorable prognosis in cancer cases | Campanella et al. (2015) |
| Heat shock proteins 70 | Breast cancer | Elevated expression of Heat Shock Proteins (HSPs) is indicative of an unfavorable prognosis in cancer cases | Gobbo et al. (2016) |
| miR-105 | Breast cancer | A candidate of regulator of cancer cell migration when upregulated expression | Zhou et al. (2014) |
| miR-10b | Breast cancer | Linked to the development of malignant traits when upregulated expression | Anfossi et al. (2014) |
| miR-1229, let-7a | Colon cancer | A candidate of up-regulator of primary colon cancer | Sugimachi et al. (2015) |
| miR-125b, 130b, 155 | Prostatic carcinoma | Promotes the development of cancerous changes when upregulated expression | Abd Elmageed et al. (2014) |
| miR-135b | Myeloma | Markedly increased in exosomes derived from HR-MM and boosts the formation of endothelial tubes | Umezu et al. (2014) |
| miR-19a | Breast cancer | A potential biomarker when upregulated expression | Anfossi et al. (2014) |
| miR-210 | Leukemia | Elevated in exosomes and amplifies endothelial migration and tube formation | Tadokoro et al. (2013) |
| miR-214 | Ovarian cancer | A candidate of up regulator of ovarian cancer when upregulated expression | Taylor and Gercel-Taylor (2008) |
| miR-223 | Breast cancer | Stimulates the invasion of breast cancer cells when upregulated expression | Yang et al. (2011) |
| miR-23b | Bladder cancer | A candidate of regulator of cancer cell migration when upregulated expression | Ostenfeld et al. (2014) |
| miR-29a | Lung cancer | Associate with the growth and metastasis of tumor when upregulated expression | Fabbri et al. (2012) |
| Programmed cell death 6-interacting protein | Colorectal cancer | It’s upregulated expression is a significant indicator of the metastatic process | Valcz et al. (2016) |
| Tumor susceptibility protein 101 | Breast cancer | Help to assess the number of exosomes in tumors when upregulated expression | Yang et al. (2017) |
A particularly noteworthy attribute of exosome-based diagnostics lies in their non-invasive nature. The isolation and analysis of exosomes from easily accessible bodily fluids, such as blood or urine, present a transformative approach for clinicians and researchers (Wang et al., 2014; Sohn et al., 2015). This minimally invasive method not only facilitates early cancer detection but also enables the continuous monitoring of cancer dynamics over time (Thind and Wilson, 2016). The escalating comprehension of exosomes and their cargo augurs well for revolutionizing cancer diagnostics and tailoring personalized treatment strategies. Researchers and clinicians are actively harnessing the potential of exosomes to usher in novel horizons in the detection and management of cancer (Patel et al., 2019).
The retrieval and scrutiny of exosomes from common bodily fluids represent a paradigm shift in cancer diagnostics, underscoring its pivotal role in advancing healthcare. This innovative methodology assumes particular significance due to its capacity for early cancer detection. The identification of cancers in their early stages holds immense clinical importance, as prompt recognition often correlates with higher treatment success rates and substantially improved patient outcomes (Hoshino et al., 2015; Hinestrosa et al., 2022). The accessibility of bodily fluids such as blood, urine, and saliva for exosome retrieval streamlines the diagnostic process, augmenting its potential impact on healthcare. The utilization of exosomes for early cancer detection heralds a promising era in medicine—one with the potential to transform the oncological landscape by saving lives through early intervention and enhancing treatment outcomes (Kalluri and LeBleu, 2020; Yu et al., 2021). As ongoing research continues to unravel the intricacies of exosomes, their role in advancing cancer diagnostics and personalized medicine is poised to play an increasingly pivotal role in shaping the future of oncology.
4.2 Monitoring disease progression for therapeutic targets
The isolation and analysis of exosomes offer a dynamic window into the complex landscape of cancer, as they can be collected at various stages of disease progression (Khan et al., 2012). This versatility provides invaluable insights into the entire continuum of cancer development, from its initial onset to metastasis and response to treatment. By examining the molecular cargo of exosomes over time, clinicians gain a nuanced understanding of how cancers evolve, adapt, and potentially resist therapy (Tang et al., 2020). This real-time monitoring becomes a critical tool in tailoring treatment strategies for individual patients. For example, in the early stages, exosomes may contain biomarkers indicative of tumor initiation, offering an opportunity for timely intervention (Jalalian et al., 2019). As cancer progresses, exosomes can carry information about metastatic potential, aiding in the prediction of disease spread (Dai et al., 2020). Moreover, tracking changes in exosome content during treatment can guide clinicians in optimizing therapeutic regimens, making them more effective and personalized (Corradetti et al., 2021). Ultimately, the ability to harness exosomes as dynamic indicators of cancer progression has the potential to revolutionize cancer care, leading to more precise and successful treatment approaches.
Beyond their diagnostic potential, exosomes emerge as direct therapeutic targets in the fight against cancer (Zhou et al., 2021). Exosomes offer a highly efficient method for delivering small molecules, proteins, and RNAs to target cancer cells. Additionally, manipulating exosomes derived from cancer cells provides a novel approach to disrupting key processes in tumorigenesis (Scavo et al., 2020). By inhibiting the release or interfering with the function of these exosomes, it becomes possible to impede critical facets of cancer progression (Moitra et al., 2011). For example, interfering with exosome release from cancer cells may curtail their ability to communicate with neighboring cells and create a conducive environment for tumor growth (Wu M et al., 2019). Additionally, targeting the specific functions of cancer-derived exosomes can hinder metastasis, potentially preventing the spread of cancer to distant organs (Tai et al., 2018). Furthermore, disrupting the exosome-mediated transfer of molecules that confer resistance to treatment can enhance the effectiveness of therapies (Steinbichler et al., 2019; Zeng and Fu, 2020). Generally, the therapeutic targeting of exosomes holds great promise for developing innovative strategies to combat cancer, offering hope for more effective treatments and improved patient outcomes.
In conclusion, exosomes represent a promising avenue for cancer research and diagnosis. Their unique ability to carry and transfer cancer-specific information opens new possibilities for early detection, monitoring, and personalized treatment strategies. As researchers continue to unravel the complexities of exosome biology and their role in cancer, we can anticipate groundbreaking advancements in cancer diagnostics and therapies in the years to come.
5 The limitations of exosome for drug application
While exosomes offer significant potential for drug application and delivery, they are not without their limitations, which need to be carefully considered in research and development. The key limitations of exosomes for drug application include:
5.1 Biological complexity and specific targeting
The intricate nature of exosomes, characterized by their diverse molecular cargo, poses significant challenges in harnessing them for precise drug delivery applications (McAndrews and Kalluri, 2019). Achieving consistent and effective drug loading into exosomes is a formidable task due to its inherent complexity (Mathieu et al., 2019; Kalluri and LeBleu, 2020). Ensuring that the therapeutic cargo is properly encapsulated within exosomes can be challenging, as it demands meticulous control over the encapsulation process to maintain therapeutic efficacy.
Furthermore, while exosomes possess a natural propensity to target specific cells or tissues, engineering them for precise targeting of disease sites adds a layer of complexity (He et al., 2022). The intricacies of achieving this level of precision involve modifying exosomes to recognize and bind exclusively to the intended target cells while minimizing off-target effects (Sun et al., 2020). Striking this delicate balance between specificity and avoiding unintended consequences remains a significant challenge in the development of exosome-based drug delivery systems (Chen H et al., 2021). Despite these challenges, ongoing research and innovation in exosome engineering hold great promise for advancing the field of targeted therapeutics.
5.2 Limited drug loading capacity and stability
Exosomes, while offering immense potential as drug delivery vehicles, do have limitations that must be considered in their application (Lee et al., 2023; Sadeghi et al., 2023). Their finite cargo capacity is one such constraint, which may pose challenges for drugs requiring high payloads (Jayaseelan and Arumugam, 2022; Malekian et al., 2023). Larger therapeutic molecules or those with low solubility might not be efficiently encapsulated within the limited confines of exosomes. This limitation necessitates careful selection of drugs to ensure compatibility with exosome-based delivery systems (Zhang L et al., 2021; Wu et al., 2022). Another concern is the fragility of exosomes. They can be susceptible to degradation, particularly when exposed to extreme temperatures or mechanical stress (Chen H et al., 2023; Zhang M et al., 2023). This fragility underscores the importance of maintaining proper storage and transportation conditions to preserve their integrity and functionality (Sanz-Ros et al., 2022). Ensuring stability throughout these processes can be a significant logistical challenge, but it is crucial for the successful deployment of exosome-based therapeutics.
In addressing these limitations, ongoing research focuses on optimizing exosome-based drug delivery by exploring innovative loading techniques, enhancing cargo capacity, and developing improved storage and transportation protocols. Despite these challenges, the versatility and potential of exosomes as drug carriers make them an exciting avenue for the future of targeted and personalized medicine.
5.3 Biological barriers and immunogenicity
When administered in vivo, exosomes encounter a series of biological barriers (e.g., including the blood-brain barrier, blood–cerebrospinal fluid barrier, blood–retinal barrier, and gastrointestinal tract) that must be overcome to effectively reach their intended target sites (Elliott and He, 2021). These barriers encompass the complex milieu of bodily fluids and tissues, which can hinder the distribution and efficacy of exosomes. Their journey through the circulatory system, extracellular matrix, and cellular barriers necessitates a thorough understanding of their interactions within these environments (Lakhal and Wood, 2011; Das et al., 2019).
Moreover, the immunogenicity of exosomes remains a topic of ongoing research and scrutiny. While they are generally considered to have low immunogenic potential, there are still gaps in our understanding (Zhao et al., 2022). Concerns about potential immune reactions to exosomes used for drug delivery persist. Undesirable immune responses could not only reduce the therapeutic efficacy of the delivered cargo but also trigger adverse effects in the recipient (Gyorgy et al., 2015). Therefore, meticulous investigation into the immune aspects of exosome-based therapies is essential for their safe and effective implementation in clinical settings.
5.4 Scale-up, production and cost
The large-scale production of exosomes with consistent quality presents significant challenges in terms of both technical feasibility and cost-effectiveness (Maumus et al., 2020). Establishing scalable manufacturing processes for exosome production continues to be a paramount concern within the field of exosome-based therapies. The complexity of isolating and purifying exosomes, coupled with the need for customization for specific therapeutic applications, can drive up production costs considerably (Sadeghi et al., 2023). The cost-intensive nature of exosome production has the potential to impact the affordability and accessibility of exosome-based treatments. As healthcare systems and patients alike seek cost-effective solutions, it becomes essential to develop more efficient and cost-efficient production methods (Ahn et al., 2022). This will not only make exosome therapies more financially accessible but also enable wider adoption within the medical community.
Recently, research endeavors have been dedicated to streamlining and optimizing exosome production processes, seeking ways to reduce costs without compromising quality, and ensuring that exosome-based treatments can reach a broader spectrum of patients in need. Achieving these goals will be pivotal in realizing the full therapeutic potential of exosomes.
6 The advantage of exosomes for cancer early detection
Studying exosomes can lead to the identification of novel biomarkers and therapeutic targets, advancing our understanding of cancer biology (Eassa, 2023). Importantly, Exosomes offer several advantages for cancer early detection, making them a promising avenue for improving the accuracy and effectiveness of diagnostic methods. For example, Exosomes can be collected at multiple time points, enabling dynamic monitoring of disease progression and treatment response (Chanteloup et al., 2020). Changes in the molecular cargo of exosomes over time can guide treatment adjustments, providing a real-time view of the disease (Sharma et al., 2017; Armstrong et al., 2018). The key advantages of using exosomes for cancer early detection include:
6.1 Specificity and high sensitivity
Exosome-based assays represent a significant advancement in cancer diagnostics due to their exceptional specificity and sensitivity (Salciccia et al., 2023). These assays are adept at detecting cancer-specific molecules, which greatly reduces the likelihood of false positives. This precision not only minimizes unnecessary diagnostic procedures but also alleviates the anxiety often associated with false alarms, ensuring a more patient-centric approach to the healthcare (Logozzi et al., 2023). Logzzi et al. reported that specific prostate-specific antigen (PSA) exosomes efficiently differentiated between prostate cancer (PC) and non-PC patients (benign prostatic hyperplasia (BPH) and healthy controls), outperforming the traditional serum PSA test. IC-ELISA achieved 98.57% sensitivity and 80.28% specificity in PC vs. BPH discrimination. Combining IC-ELISA with NFSC increased sensitivity to 96% and specificity to 100% (Logozzi et al., 2019).
What sets exosome-based assays apart is their capacity to detect even trace amounts of cancer biomarkers, even when they are present in low concentrations (Saad et al., 2021). This heightened sensitivity becomes a potent tool in the early detection of cancer, potentially identifying the disease before clinical symptoms manifest (Hu et al., 2022). This early detection, because of exosome-based assays, substantially enhances the chances of successful treatment outcomes, ultimately improving patient prognosis.
Moreover, exosomes offer a more comprehensive representation of the entire tumor’s molecular profile compared to traditional biopsies, where only a small portion of the tumor is sampled (Yu et al., 2021). This advantage significantly reduces the potential for sampling bias and ensures that clinicians have a more accurate and holistic understanding of the disease (Li L et al., 2023). In essence, exosome-based assays usher in a new era of cancer diagnostics, characterized by enhanced accuracy, sensitivity, and patient-centered care.
6.2 Non-invasive sampling and patient-friendly
Exosomes represent a groundbreaking avenue for non-invasive diagnostics as they can be conveniently isolated from readily accessible bodily fluids, including blood, urine, saliva, and cerebrospinal fluid (Lin et al., 2015; Yi et al., 2022). This non-invasive sampling method eliminates the need for painful and invasive procedures such as biopsies, which can be uncomfortable and entail inherent risks (Chen J et al., 2021). Consequently, exosome-based tests offer a patient-friendly approach, requiring nothing more than simple blood or urine collection (Liu et al., 2018). This simplicity in sampling not only enhances patient comfort but also encourages a broader spectrum of individuals to undergo regular screenings, contributing significantly to early cancer detection efforts.
Furthermore, the molecular cargo of exosomes can be meticulously analyzed to identify specific cancer types and discern their unique characteristics (Mitchell et al., 2022). This valuable information provides a foundation for the development of personalized treatment plans tailored to the patient’s specific cancer subtype (Srivastava et al., 2018). Recent studies demonstrated that exosomes from lung tumor cells reflected the genetic mutations of the parent cell lines, notably EGFR status (Thakur et al., 2014). In a separate in vitro study, exosomes from various cancer cell lines facilitated the transfer of activated EGFR to endothelial cells, triggering MAPK and AKT pathways (Al-Nedawi et al., 2009). Therefore, specific target methods can be developed in precise treatment strategies not only to improve therapeutic efficacy but also to minimize potential side effects, enhancing the overall quality of care and patient outcomes (Sancho-Albero et al., 2019; He et al., 2022). In summary, exosomes hold significant promise for cancer early detection due to their non-invasive nature, ability to detect cancer-specific biomarkers, high sensitivity, and potential for dynamic monitoring. Moreover, exosome-based diagnostics represent a transformative shift towards patient-friendly and personalized approaches to cancer detection and treatment.
7 Advanced strategies and technologies in exosome-based cancer detection
In the pursuit of harnessing the potential of exosomes to improve efficiency and precise cancer detection, several up-to-date innovative strategies and cutting-edge technologies have been developed (Chen J et al., 2021; Cheng et al., 2022). These approaches have the power to transform cancer diagnostics, providing more precise, non-invasive, and early detection methods. The key strategies and technologies that are paving the way for the utilization of exosomes in cancer detection.
7.1 Liquid biopsies for revolutionizing cancer diagnosis
Liquid biopsies are a groundbreaking strategy in cancer detection, utilizing the non-invasive collection and analysis of exosomes from body fluids like blood, urine, and saliva (Shegekar et al., 2023). This technique is a significant evolution from traditional tissue biopsies, which are often invasive and not always suitable for all patients. Exosomes, when released by cancer cells into the bloodstream, contain critical biomarkers (mutated DNA, proteins, microRNAs) that offer real-time insights into tumor status, are more convenient and authentic than the circulating tumor DNA (ctDNA) and circulating tumor cells (CTCs) (Heitzer et al., 2019; Yu et al., 2022). For example, the abundance of exosomes, with concentrations around 10^9 particles per milliliter in biological fluids, facilitates the collection of these vesicles. In contrast, only a few circulating tumor cells (CTCs) are present in 1 mL of blood samples (Cai et al., 2015). On the other hand, due to their lipid bilayer composition, exosomes inherently possess stability, enabling them to circulate consistently under physiological conditions, even within the rigorous conditions of the tumor microenvironment. This intrinsic biological stability facilitates the prolonged preservation of samples for the isolation and detection of exosomes (Yu et al., 2021). Therefore, liquid biopsies on exosomes may facilitate early cancer detection, potentially before symptoms appear, and allow continuous monitoring of disease progression and treatment response. This method enhances early detection capabilities and provides a less invasive alternative to conventional biopsies.
7.2 Nanotechnology for precision and sensitivity in detection
Nanotechnology has been instrumental in developing exosome-based biosensors and platforms for the sensitive detection of cancer-associated biomarkers (Mukhtar et al., 2021). Nanoparticles and nanomaterials are used to isolate exosomes from biological samples accurately, ensuring precise detection of cancer-related exosomal cargo. These nano-based methods increase the sensitivity of assays, enabling the detection of minimal biomarker levels, thus improving the accuracy of cancer diagnoses and aiding in identifying specific cancer types and molecular signatures. For example, Patolsky et al. developed a nanotechnology-based method for DNA detection, which has the potential for cancer diagnosis. They achieved this by integrating biotin labels into DNA replicas. These replicas were attached to magnetic particles, forming a nanodevice. This innovative approach enhances the accuracy of DNA-based detection in cancer diagnostics (Patolsky et al., 2003). Similarly, the research team led by Xu made significant advancements in the field of nanoparticle technology for cancer diagnosis. They created shell-engineered nanoparticles (NPs) that were coated with silver (Ag) and gold (Au). This coating substantially improved the sensitivity of PCR-based DNA detection methods which can be used in identifying cancer (Zhao et al., 2014).
7.3 Machine learning and bioinformatics for interpreting complex data
The vast and complex data from exosome profiling necessitate advanced analysis tools, where machine learning and bioinformatics play a crucial role (Chen J et al., 2023). Machine learning algorithms are adept at identifying patterns in large datasets, making them ideal for interpreting exosome profiles (Li J et al., 2023). Li et al. present a machine learning approach for non-invasive cancer diagnosis using exosome protein markers, achieving high accuracy in identifying cancer types with an advanced biomarker signature and sophisticated data models, marking a significant leap in early cancer detection methodologies (Li B et al., 2023). Wang et al. employed the Least Absolute Shrinkage and Selection Operator (LASSO) regression algorithm to construct a prognostic model based on differentially expressed genes (DEGs). The model’s predictive accuracy and sensitivity were validated through prognostic analysis and receiver operating characteristic curve analysis (Wang Z et al., 2023). These algorithms can differentiate between cancerous and non-cancerous samples, classify cancer subtypes, and predict treatment outcomes. Additionally, bioinformatics tools are essential for managing and interpreting the extensive data from exosome analysis, helping to derive meaningful insights from the complex molecular profiles of exosomes.
The integration of liquid biopsies, nanotechnology, machine learning, and bioinformatics represents a paradigm shift in cancer diagnostics. This convergence facilitates non-invasive, precise early cancer detection, and dynamic disease monitoring through exosome analysis. The precision of nanotechnology and the analytical power of machine learning and bioinformatics in interpreting complex data highlight the transformative potential of exosomes in cancer detection. As these technologies continue to evolve, exosome-based cancer detection is poised to become a fundamental aspect of early diagnosis and personalized cancer treatment, significantly impacting oncology by improving patient outcomes and expanding our understanding of cancer biology.
8 Prospective and conclusion
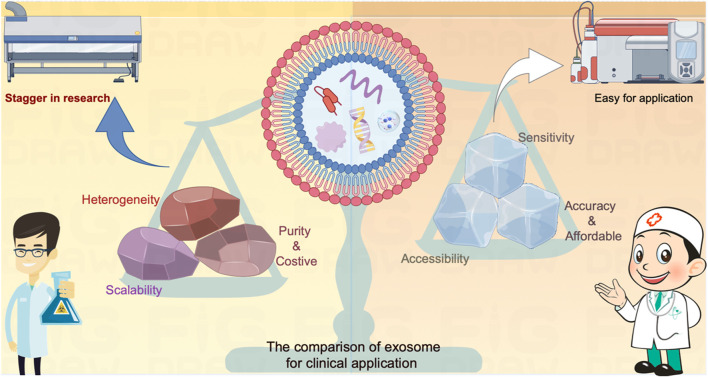
Exosomes hold immense potential for cancer detection and are more practicable for achieving compared to cancer treatment per the accessibility in the clinical applications (Figure 3). However, several critical challenges need to be confronted to fully harness their potential: A pressing concern is the absence of standardized protocols for exosome isolation, characterization, and analysis. The establishment of rigorous standards is imperative to ensure the reproducibility and reliability of exosome-based assays across various laboratories and therapeutic companies. Standardization efforts will bolster the credibility of exosome-based diagnostics. Moreover, the success of exosome-based assays hinges on achieving heightened specificity and sensitivity. The aim is to minimize false positives and false negatives, which can have significant clinical implications. This necessitates the development of more selective and reliable biomarkers, as well as advancements in detection technologies.
FIGURE 3.
The comparison of exosome on diagnosis versus treatment for clinical application. The exosome serves as a carrier for various contents that actively influence cellular function or provide real-time insights into the developmental processes of target cells. These attributes have demonstrated significant value in the realm of cancer diagnosis and treatment. Nevertheless, the direct utilization of exosomes as mediators for cancer treatment faces inherent limitations due to contemporary technical challenges, encompassing issues related to heterogeneity, scalability, and purity. These constraints impede the seamless translation of exosome-based therapies from laboratory research to clinical applications. In contrast, leveraging exosomes as sensors offers a promising alternative. By repurposing them into highly sensitive, easily accessible, accurate, and cost-effective tools, these challenges can be transformed into opportunities for detecting cancers. The incorporation of state-of-the-art diagnostic equipment facilitates a more expeditious and practical application of exosomes in cancer diagnosis compared to treatment methodologies.
On the other hand, exosomes exhibit great promise in the research field which has a well-controlled environment, but their real-world utility as cancer biomarkers needs to be rigorously validated through large-scale clinical trials. These trials are essential to establish the relevance and efficacy of exosome-based diagnostics. Demonstrating their performance in diverse patient populations is a crucial step toward clinical application. In addition, the ethical dimensions of employing exosomes for cancer detection require rigid contemplation. Therefore, obtaining informed consent from patients, ensuring data privacy, and adhering to ethical guidelines are very important. Safeguarding patient rights and privacy is an ethical requirement in the development and application of exosome-based diagnostics for cancer.
In conclusion, exosomes represent a promising avenue for cancer detection because they are enriched with cancer-specific biomolecules, and offer non-invasive, sensitive, and specific avenues to identify cancer-related markers. As research continues to advance, exosome-based assays can hold the potential to revolutionize cancer diagnosis, facilitating earlier detection and improving patient survival and life quality. Nevertheless, addressing the challenges associated with standardization, specificity, sensitivity, clinical validation, and ethical considerations is significant to comprehensively realize the translational potential of exosomes for cancer detection in clinical practice.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work is supported by the National Natural Science Foundation of China (No. 8197211).
Author contributions
ZW: Writing–original draft. QW: Visualization, Writing–review and editing. FQ: Writing–review and editing. JC: Conceptualization, Project administration, Resources, Supervision, Writing–review and editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abd Elmageed Z. Y., Yang Y., Thomas R., Ranjan M., Mondal D., Moroz K., et al. (2014). Neoplastic reprogramming of patient-derived adipose stem cells by prostate cancer cell-associated exosomes. Stem Cells 32 (4), 983–997. 10.1002/stem.1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnoletto C., Pignochino Y., Caruso C., Garofalo C. (2023). Exosome-based liquid biopsy approaches in bone and soft tissue sarcomas: review of the literature, prospectives, and hopes for clinical application. Int. J. Mol. Sci. 24 (6), 5159. 10.3390/ijms24065159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S. H., Ryu S. W., Choi H., You S., Park J., Choi C. (2022). Manufacturing therapeutic exosomes: from bench to industry. Mol. Cells 45 (5), 284–290. 10.14348/molcells.2022.2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksandrowicz K., Hempel D., Politynska B., Wojtukiewicz A. M., Honn K. V., Tang D. G., et al. (2023). The complex role of thrombin in cancer and metastasis: focus on interactions with the immune system. Semin. Thromb. Hemost. 2023, 1776875. 10.1055/s-0043-1776875 [DOI] [PubMed] [Google Scholar]
- Ali S. A., Huang M. B., Campbell P. E., Roth W. W., Campbell T., Khan M., et al. (2010). Genetic characterization of HIV type 1 Nef-induced vesicle secretion. AIDS Res. Hum. Retroviruses 26 (2), 173–192. 10.1089/aid.2009.0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Nedawi K., Meehan B., Kerbel R. S., Allison A. C., Rak J. (2009). Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc. Natl. Acad. Sci. U. S. A. 106 (10), 3794–3799. 10.1073/pnas.0804543106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Nedawi K., Meehan B., Micallef J., Lhotak V., May L., Guha A., et al. (2008). Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 10 (5), 619–624. 10.1038/ncb1725 [DOI] [PubMed] [Google Scholar]
- Alonso-Curbelo D., Riveiro-Falkenbach E., Perez-Guijarro E., Cifdaloz M., Karras P., Osterloh L., et al. (2014). RAB7 controls melanoma progression by exploiting a lineage-specific wiring of the endolysosomal pathway. Cancer Cell 26 (1), 61–76. 10.1016/j.ccr.2014.04.030 [DOI] [PubMed] [Google Scholar]
- Altintas O., Saylan Y. (2023). Exploring the versatility of exosomes: a review on isolation, characterization, detection methods, and diverse applications. Anal. Chem. 95 (44), 16029–16048. 10.1021/acs.analchem.3c02224 [DOI] [PubMed] [Google Scholar]
- Amini A., Verma V., Simone C. B., 2nd, Chetty I. J., Chun S. G., Donington J., et al. (2022). American radium society appropriate use criteria for radiation therapy in oligometastatic or oligoprogressive non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 112 (2), 361–375. 10.1016/j.ijrobp.2021.09.022 [DOI] [PubMed] [Google Scholar]
- Anel A., Gallego-Lleyda A., de Miguel D., Naval J., Martinez-Lostao L. (2019). Role of exosomes in the regulation of T-cell mediated immune responses and in autoimmune disease. Cells 8 (2), 154. 10.3390/cells8020154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anfossi S., Giordano A., Gao H., Cohen E. N., Tin S., Wu Q., et al. (2014). High serum miR-19a levels are associated with inflammatory breast cancer and are predictive of favorable clinical outcome in patients with metastatic HER2+ inflammatory breast cancer. PLoS One 9 (1), e83113. 10.1371/journal.pone.0083113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenaccio C., Chiozzini C., Columba-Cabezas S., Manfredi F., Affabris E., Baur A., et al. (2014). Exosomes from human immunodeficiency virus type 1 (HIV-1)-infected cells license quiescent CD4+ T lymphocytes to replicate HIV-1 through a Nef- and ADAM17-dependent mechanism. J. Virol. 88 (19), 11529–11539. 10.1128/JVI.01712-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong E. A., Beal E. W., Chakedis J., Paredes A. Z., Moris D., Pawlik T. M., et al. (2018). Exosomes in pancreatic cancer: from early detection to treatment. J. Gastrointest. Surg. 22 (4), 737–750. 10.1007/s11605-018-3693-1 [DOI] [PubMed] [Google Scholar]
- Barile L., Vassalli G. (2017). Exosomes: therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 174, 63–78. 10.1016/j.pharmthera.2017.02.020 [DOI] [PubMed] [Google Scholar]
- Bassani S., Lee Y. K., Campagnari V., Eccher A., Monzani D., Nocini R., et al. (2023). From hype to reality: a narrative review on the promising role of artificial intelligence in larynx cancer detection and transoral microsurgery. Crit. Rev. Oncog. 28 (3), 21–24. 10.1615/CritRevOncog.2023049134 [DOI] [PubMed] [Google Scholar]
- Bernard M. A., Zhao H., Yue S. C., Anandaiah A., Koziel H., Tachado S. D. (2014). Novel HIV-1 miRNAs stimulate TNFα release in human macrophages via TLR8 signaling pathway. PLoS One 9 (9), e106006. 10.1371/journal.pone.0106006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdag G., Mumusoglu S., Zengin D., Karabulut E., Yildiz B. O. (2016). The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum. Reprod. 31 (12), 2841–2855. 10.1093/humrep/dew218 [DOI] [PubMed] [Google Scholar]
- Butreddy A., Kommineni N., Dudhipala N. (2021). Exosomes as naturally occurring vehicles for delivery of biopharmaceuticals: insights from drug delivery to clinical perspectives. Nanomater. (Basel) 11 (6), 1481. 10.3390/nano11061481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X., Janku F., Zhan Q., Fan J. B. (2015). Accessing genetic information with liquid biopsies. Trends Genet. 31 (10), 564–575. 10.1016/j.tig.2015.06.001 [DOI] [PubMed] [Google Scholar]
- Cambier L. D., Stachelek K., Triska M., Jubran R., Huang M. Y., Li W. Y., et al. (2021). Extracellular vesicle-associated repetitive element DNAs as candidate osteosarcoma biomarkers. Sci. Rep. 11 (1), 94. 10.1038/s41598-020-77398-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella C., Rappa F., Sciumè C., Marino Gammazza A., Barone R., Bucchieri F., et al. (2015). Heat shock protein 60 levels in tissue and circulating exosomes in human large bowel cancer before and after ablative surgery. Cancer 121 (18), 3230–3239. 10.1002/cncr.29499 [DOI] [PubMed] [Google Scholar]
- Chanteloup G., Cordonnier M., Isambert N., Bertaut A., Hervieu A., Hennequin A., et al. (2020). Monitoring HSP70 exosomes in cancer patients' follow up: a clinical prospective pilot study. J. Extracell. Vesicles 9 (1), 1766192. 10.1080/20013078.2020.1766192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che X., Jian F., Chen C., Liu C., Liu G., Feng W. (2020). PCOS serum-derived exosomal miR-27a-5p stimulates endometrial cancer cells migration and invasion. J. Mol. Endocrinol. 64 (1), 1–12. 10.1530/JME-19-0159 [DOI] [PubMed] [Google Scholar]
- Chen B., Li M. Y., Guo Y., Zhao X., Lim H. C. (2017). Mast cell-derived exosomes at the stimulated acupoints activating the neuro-immune regulation. Chin. J. Integr. Med. 23 (11), 878–880. 10.1007/s11655-016-2269-8 [DOI] [PubMed] [Google Scholar]
- Chen H., Wang L., Zeng X., Schwarz H., Nanda H. S., Peng X., et al. (2021). Exosomes, a new star for targeted delivery. Front. Cell Dev. Biol. 9, 751079. 10.3389/fcell.2021.751079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Yao H., Chi J., Li C., Liu Y., Yang J., et al. (2023). Engineered exosomes as drug and RNA co-delivery system: new hope for enhanced therapeutics? Front. Bioeng. Biotechnol. 11, 1254356. 10.3389/fbioe.2023.1254356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Dong H., Fu R., Liu X., Xue F., Liu W., et al. (2023). Machine learning analyses constructed a novel model to predict recurrent thrombosis in adults with essential thrombocythemia. J. Thromb. Thrombolysis 56 (2), 291–300. 10.1007/s11239-023-02833-7 [DOI] [PubMed] [Google Scholar]
- Chen J., Li P., Zhang T., Xu Z., Huang X., Wang R., et al. (2021). Review on strategies and technologies for exosome isolation and purification. Front. Bioeng. Biotechnol. 9, 811971. 10.3389/fbioe.2021.811971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Yang Q., Wang R., Luo R., Zhu S., Li M., et al. (2022). Emerging advances of detection strategies for tumor-derived exosomes. Int. J. Mol. Sci. 23 (2), 868. 10.3390/ijms23020868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y. C., Wei W. C., Huang S. H., Shih C. M., Hsu C. P., Chang K. J., et al. (2014). Rab11 regulates E-cadherin expression and induces cell transformation in colorectal carcinoma. BMC Cancer 14, 587. 10.1186/1471-2407-14-587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradetti B., Gonzalez D., Mendes Pinto I., Conlan R. S. (2021). Editorial: exosomes as therapeutic systems. Front. Cell Dev. Biol. 9, 714743. 10.3389/fcell.2021.714743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J., Su Y., Zhong S., Cong L., Liu B., Yang J., et al. (2020). Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct. Target Ther. 5 (1), 145. 10.1038/s41392-020-00261-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das C. K., Jena B. C., Banerjee I., Das S., Parekh A., Bhutia S. K., et al. (2019). Exosome as a novel shuttle for delivery of therapeutics across biological barriers. Mol. Pharm. 16 (1), 24–40. 10.1021/acs.molpharmaceut.8b00901 [DOI] [PubMed] [Google Scholar]
- de Carvalho J. V., de Castro R. O., da Silva E. Z. M., Silveira P. P., da Silva-Januario M. E., Arruda E., et al. (2014). Nef neutralizes the ability of exosomes from CD4+ T cells to act as decoys during HIV-1 infectionT Cells to Act as Decoys during HIV-1 Infection. PLoS One 9 (11), e113691. 10.1371/journal.pone.0113691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Pena H., Madrigal J. A., Rusakiewicz S., Bencsik M., Cave G. W., Selman A., et al. (2009). Artificial exosomes as tools for basic and clinical immunology. J. Immunol. Methods 344 (2), 121–132. 10.1016/j.jim.2009.03.011 [DOI] [PubMed] [Google Scholar]
- Eassa H. A. (2023). Exosomes: double-edged weapon in cancer therapy. Curr. Pharm. Des. 29 (30), 2366–2368. 10.2174/0113816128272352231013074525 [DOI] [PubMed] [Google Scholar]
- Elliott R. O., He M. (2021). Unlocking the power of exosomes for crossing biological barriers in drug delivery. Pharmaceutics 13 (1), 122. 10.3390/pharmaceutics13010122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri M., Paone A., Calore F., Galli R., Gaudio E., Santhanam R., et al. (2012). MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. U. S. A. 109 (31), E2110–E2116. 10.1073/pnas.1209414109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C., She J., Chen X., Zhang Q., Zhang X., Wang Y., et al. (2019). Exosomal miR-196a-1 promotes gastric cancer cell invasion and metastasis by targeting SFRP1. Nanomedicine (Lond) 14 (19), 2579–2593. 10.2217/nnm-2019-0053 [DOI] [PubMed] [Google Scholar]
- Feng K., Xing Z., Dai Q., Cheng H., Wang X. (2023). Role of aggressive locoregional surgery in treatment strategies for ipsilateral supraclavicular lymph node metastasis of breast cancer: a real-world cohort study. Front. Mol. Biosci. 10, 1248410. 10.3389/fmolb.2023.1248410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadaran P., Gunassekaran G. R., Rajendran R. L., Oh J. M., Vadevoo S. M. P., Lee H. W., et al. (2022a). Interleukin-4 receptor targeting peptide decorated extracellular vesicles as a platform for in vivo drug delivery to thyroid cancer. Biomedicines 10 (8), 1978. 10.3390/biomedicines10081978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadaran P., Madhyastha H., Madhyastha R., Rajendran R. L., Nakajima Y., Watanabe N., et al. (2022b). The emerging role of exosomes in innate immunity, diagnosis and therapy. Front. Immunol. 13, 1085057. 10.3389/fimmu.2022.1085057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadaran P., Rajendran R. L., Kwack M. H., Jeyaraman M., Hong C. M., Sung Y. K., et al. (2022c). Application of cell-derived extracellular vesicles and engineered nanovesicles for hair growth: from mechanisms to therapeutics. Front. Cell Dev. Biol. 10, 963278. 10.3389/fcell.2022.963278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Zhang F., Zhou Q., Xu H., Bian J. (2023). Metastasis, characteristic, and treatment of breast cancer in young women and older women: a study from the Surveillance, Epidemiology, and End Results registration database. PLoS One 18 (11), e0293830. 10.1371/journal.pone.0293830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Romero N., Carrión-Navarro J., Esteban-Rubio S., Lázaro-Ibáñez E., Peris-Celda M., Alonso M. M., et al. (2017). DNA sequences within glioma-derived extracellular vesicles can cross the intact blood-brain barrier and be detected in peripheral blood of patients. Oncotarget 8 (1), 1416–1428. 10.18632/oncotarget.13635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X., Qi Y., Liu X., Shi Y., Li H., Zhao L. (2022). A multifunctional antibacterial and self-healing hydrogel laden with bone marrow mesenchymal stem cell-derived exosomes for accelerating diabetic wound healing. Biomater. Adv. 133, 112613. 10.1016/j.msec.2021.112613 [DOI] [PubMed] [Google Scholar]
- Gera K., Kahramangil D., Fenton G. A., Martir D., Rodriguez D. N., Ijaz Z., et al. (2023). Prognosis and treatment outcomes of bone metastasis in gallbladder adenocarcinoma: a SEER-based study. Cancers (Basel) 15 (20), 5055. 10.3390/cancers15205055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbo J., Marcion G., Cordonnier M., Dias A. M. M., Pernet N., Hammann A., et al. (2016). Restoring anticancer immune response by targeting tumor-derived exosomes with a HSP70 peptide aptamer. J. Natl. Cancer Inst. 108 (3), djv330. 10.1093/jnci/djv330 [DOI] [PubMed] [Google Scholar]
- Greening D. W., Gopal S. K., Xu R., Simpson R. J., Chen W. (2015). Exosomes and their roles in immune regulation and cancer. Semin. Cell Dev. Biol. 40, 72–81. 10.1016/j.semcdb.2015.02.009 [DOI] [PubMed] [Google Scholar]
- Gudbergsson J. M., Johnsen K. B. (2019). Exosomes and autophagy: rekindling the vesicular waste hypothesis. J. Cell Commun. Signal 13 (4), 443–450. 10.1007/s12079-019-00524-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorgy B., Hung M. E., Breakefield X. O., Leonard J. N. (2015). Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu. Rev. Pharmacol. Toxicol. 55, 439–464. 10.1146/annurev-pharmtox-010814-124630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H., Matsumoto T. (2018). Mechanisms of tolerance induction by dendritic cells in vivo . Front. Immunol. 9, 350. 10.3389/fimmu.2018.00350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Kitago M., Abe Y., Yagi H., Hasegawa Y., Hori S., et al. (2023). Long-term survival after surgical resection for bone metastasis from pancreatic cancer: a case report. Med. Baltim. 102 (46), e35856. 10.1097/MD.0000000000035856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Ren W., Wang W., Han W., Jiang L., Zhang D., et al. (2022). Exosomal targeting and its potential clinical application. Drug Deliv. Transl. Res. 12 (10), 2385–2402. 10.1007/s13346-021-01087-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He N. H., Liu M., Hsu J., Xue Y. H., Chou S., Burlingame A., et al. (2010). HIV-1 tat and host AFF4 recruit two transcription elongation factors into a bifunctional complex for coordinated activation of HIV-1 transcription. Mol. Cell 38 (3), 428–438. 10.1016/j.molcel.2010.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzer E., Haque I. S., Roberts C. E. S., Speicher M. R. (2019). Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 20 (2), 71–88. 10.1038/s41576-018-0071-5 [DOI] [PubMed] [Google Scholar]
- Hinestrosa J. P., Kurzrock R., Lewis J. M., Schork N. J., Schroeder G., Kamat A. M., et al. (2022). Early-stage multi-cancer detection using an extracellular vesicle protein-based blood test. Commun. Med. (Lond) 2, 29. 10.1038/s43856-022-00088-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holladay L., Luu J., Balendra V., Kmetz K. (2023). Current and potential treatment of colorectal cancer metastasis to bone. Cancer Treat. Res. Commun. 37, 100763. 10.1016/j.ctarc.2023.100763 [DOI] [PubMed] [Google Scholar]
- Hoshino A., Costa-Silva B., Shen T. L., Rodrigues G., Hashimoto A., Tesic Mark M., et al. (2015). Tumour exosome integrins determine organotropic metastasis. Nature 527 (7578), 329–335. 10.1038/nature15756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu D. H., Paz P., Villaflor G., Rivas A., Mehta-Damani A., Angevin E., et al. (2003). Exosomes as a tumor vaccine: enhancing potency through direct loading of antigenic peptides. J. Immunother. 26 (5), 440–450. 10.1097/00002371-200309000-00007 [DOI] [PubMed] [Google Scholar]
- Hu C., Jiang W., Lv M., Fan S., Lu Y., Wu Q., et al. (2022). Potentiality of exosomal proteins as novel cancer biomarkers for liquid biopsy. Front. Immunol. 13, 792046. 10.3389/fimmu.2022.792046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T., Chang S., Qi F., Zhang Z., Chen J., Jiang L., et al. (2023). Neural grafts containing exosomes derived from Schwann cell-like cells promote peripheral nerve regeneration in rats. Burns Trauma 11, tkad013. 10.1093/burnst/tkad013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Yang S., Xu Z., Zhang X., Wang H., Fan G., et al. (2023). Prevalence and risk factors of bone metastasis and the development of bone metastatic prognostic classification system: a pan-cancer population study. Aging (Albany NY) 15, 13134–13149. 10.18632/aging.205224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun K. A., Gwak H., Lee J., Kwak B., Jung H. I. (2018). Salivary exosome and cell-free DNA for cancer detection. Micromachines (Basel) 9 (7), 340. 10.3390/mi9070340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalalian S. H., Ramezani M., Jalalian S. A., Abnous K., Taghdisi S. M. (2019). Exosomes, new biomarkers in early cancer detection. Anal. Biochem. 571, 1–13. 10.1016/j.ab.2019.02.013 [DOI] [PubMed] [Google Scholar]
- Jayaseelan V. P., Arumugam P. (2022). Exosome engineering: a promising step toward cancer therapeutics. Epigenomics 14 (9), 503–506. 10.2217/epi-2021-0474 [DOI] [PubMed] [Google Scholar]
- Ji H., Greening D. W., Barnes T. W., Lim J. W., Tauro B. J., Rai A., et al. (2013). Proteome profiling of exosomes derived from human primary and metastatic colorectal cancer cells reveal differential expression of key metastatic factors and signal transduction components. Proteomics 13 (10-11), 1672–1686. 10.1002/pmic.201200562 [DOI] [PubMed] [Google Scholar]
- Jiang C., Zhang N., Hu X., Wang H. (2021). Tumor-associated exosomes promote lung cancer metastasis through multiple mechanisms. Mol. Cancer 20 (1), 117. 10.1186/s12943-021-01411-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C., Wu P., Li L., Xu W., Qian H. (2021). Exosomes: emerging therapy delivery tools and biomarkers for kidney diseases. Stem Cells Int. 2021, 7844455. 10.1155/2021/7844455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakiuchi Y., Kuroda S., Kanaya N., Kagawa S., Tazawa H., Fujiwara T. (2023). Exosomes as a drug delivery tool for cancer therapy: a new era for existing drugs and oncolytic viruses. Expert Opin. Ther. Targets 27 (9), 807–816. 10.1080/14728222.2023.2259102 [DOI] [PubMed] [Google Scholar]
- Kalluri R., LeBleu V. S. (2020). The biology, function, and biomedical applications of exosomes. Science 367 (6478), eaau6977. 10.1126/science.aau6977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski V. L., Ellwanger J. H., Chies J. A. B. (2019). Extracellular vesicles in host-pathogen interactions and immune regulation - exosomes as emerging actors in the immunological theater of pregnancy. Heliyon 5 (8), e02355. 10.1016/j.heliyon.2019.e02355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C., He H., Liu P., Liu Y., Li X., Zhang J., et al. (2023). Role of dendritic cell-derived exosomes in allergic rhinitis (Review). Int. J. Mol. Med. 52 (6), 117. 10.3892/ijmm.2023.5320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada T., Shim S. R., Quhal F., Rajwa P., Pradere B., Yanagisawa T., et al. (2023). Diagnostic accuracy of liquid biomarkers for clinically significant prostate cancer detection: a systematic review and diagnostic meta-analysis of multiple thresholds. Eur. Urol. Oncol. 2023, 29. 10.1016/j.euo.2023.10.029 [DOI] [PubMed] [Google Scholar]
- Keseru J. S., Soltesz B., Lukacs J., Marton E., Szilagyi-Bonizs M., Penyige A., et al. (2019). Detection of cell-free, exosomal and whole blood mitochondrial DNA copy number in plasma or whole blood of patients with serous epithelial ovarian cancer. J. Biotechnol. 298, 76–81. 10.1016/j.jbiotec.2019.04.015 [DOI] [PubMed] [Google Scholar]
- Khan S., Jutzy J. M., Valenzuela M. M., Turay D., Aspe J. R., Ashok A., et al. (2012). Plasma-derived exosomal survivin, a plausible biomarker for early detection of prostate cancer. PLoS One 7 (10), e46737. 10.1371/journal.pone.0046737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A., Gangadaran P., Chavda V. P., Jogalekar M. P., Muthusamy R., Valu D., et al. (2022). Convalescent serum-derived exosomes: attractive niche as COVID-19 diagnostic tool and vehicle for mRNA delivery. Exp. Biol. Med. (Maywood) 247 (14), 1244–1252. 10.1177/15353702221092984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudinov V. A., Artyushev R. I., Zurina I. M., Lapshin R. D., Snopova L. B., Mukhina I. V., et al. (2021). Antimicrobial and regenerative effects of placental multipotent mesenchymal stromal cell secretome-based chitosan gel on infected burns in rats. Pharm. (Basel) 14 (12), 1263. 10.3390/ph14121263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumata Y., Iinuma H., Suzuki Y., Tsukahara D., Midorikawa H., Igarashi Y., et al. (2018). Exosome-encapsulated microRNA-23b as a minimally invasive liquid biomarker for the prediction of recurrence and prognosis of gastric cancer patients in each tumor stage. Oncol. Rep. 40 (1), 319–330. 10.3892/or.2018.6418 [DOI] [PubMed] [Google Scholar]
- Kurakevich E., Hennet T., Hausmann M., Rogler G., Borsig L. (2013). Milk oligosaccharide sialyl(α2,3)lactose activates intestinal CD11c+ cells through TLR4. Proc. Natl. Acad. Sci. U. S. A. 110 (43), 17444–17449. 10.1073/pnas.1306322110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Z., Liang J., Zhang J., Mao Y., Zheng X., Shen X., et al. (2023). Exosomes as a delivery tool of exercise-induced beneficial factors for the prevention and treatment of cardiovascular disease: a systematic review and meta-analysis. Front. Physiol. 14, 1190095. 10.3389/fphys.2023.1190095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhal S., Wood M. J. (2011). Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. Bioessays 33 (10), 737–741. 10.1002/bies.201100076 [DOI] [PubMed] [Google Scholar]
- Lee C. S., Fan J., Hwang H. S., Kim S., Chen C., Kang M., et al. (2023). Bone-targeting exosome mimetics engineered by bioorthogonal surface functionalization for bone tissue engineering. Nano Lett. 23 (4), 1202–1210. 10.1021/acs.nanolett.2c04159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lema D. A., Burlingham W. J. (2019). Role of exosomes in tumour and transplant immune regulation. Scand. J. Immunol. 90 (5), e12807. 10.1111/sji.12807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenassi M., Cagney G., Liao M. F., Vaupotic T., Bartholomeeusen K., Cheng Y. F., et al. (2010). HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cellsT Cells. Traffic 11 (1), 110–122. 10.1111/j.1600-0854.2009.01006.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Kugeratski F. G., Kalluri R. (2023). A novel machine learning algorithm selects proteome signature to specifically identify cancer exosomes. bioRxiv. Available at: 10.1101/2023.07.18.549557. [DOI] [PMC free article] [PubMed]
- Li B., Luan S., Chen J., Zhou Y., Wang T., Li Z., et al. (2020). The MSC-derived exosomal lncRNA H19 promotes wound healing in diabetic foot ulcers by upregulating PTEN via MicroRNA-152-3p. Mol. Ther. Nucleic Acids 19, 814–826. 10.1016/j.omtn.2019.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Tang W., Yang F. (2020). Cancer liquid biopsy using integrated microfluidic exosome analysis platforms. Biotechnol. J. 15 (5), e1900225. 10.1002/biot.201900225 [DOI] [PubMed] [Google Scholar]
- Li J., Liang Y., Zhao X., Wu C. (2023). Integrating machine learning algorithms to systematically assess reactive oxygen species levels to aid prognosis and novel treatments for triple -negative breast cancer patients. Front. Immunol. 14, 1196054. 10.3389/fimmu.2023.1196054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Jay S. M., Wang Y., Wu S. W., Xiao Z. (2017). IL-12 stimulates CTLs to secrete exosomes capable of activating bystander CD8(+) T cells. Sci. Rep. 7 (1), 13365. 10.1038/s41598-017-14000-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Zhang L., Montgomery K. C., Jiang L., Lyon C. J., Hu T. Y. (2023). Advanced technologies for molecular diagnosis of cancer: state of pre-clinical tumor-derived exosome liquid biopsies. Mater Today Bio 18, 100538. 10.1016/j.mtbio.2022.100538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Zheng W. (2023). Metastasis of endometrial adenocarcinoma masquerading as a primary rectal cancer: a rare case report with literature review. Med. Baltim. 102 (46), e36170. 10.1097/MD.0000000000036170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Wang H., Peng H., Huyan T., Cacalano N. A. (2019). Exosomes: versatile nano mediators of immune regulation. Cancers (Basel) 11 (10), 1557. 10.3390/cancers11101557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Hu Y., Jiang T., Han Y., Han G., Chen J., et al. (2014). Rab27A regulates exosome secretion from lung adenocarcinoma cells A549: involvement of EPI64. APMIS 122 (11), 1080–1087. 10.1111/apm.12261 [DOI] [PubMed] [Google Scholar]
- Li X., Liu L., Chai J. (2015). Progress of mesenchymal stem cell-derived exosomes in tissue repair. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 29 (2), 234–238. [PubMed] [Google Scholar]
- Lin B., Lei Y., Wang J., Zhu L., Wu Y., Zhang H., et al. (2021). Microfluidic-based exosome analysis for liquid biopsy. Small Methods 5 (3), e2001131. 10.1002/smtd.202001131 [DOI] [PubMed] [Google Scholar]
- Lin J., Li J., Huang B., Liu J., Chen X., Chen X. M., et al. (2015). Exosomes: novel biomarkers for clinical diagnosis. ScientificWorldJournal 2015, 657086. 10.1155/2015/657086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N N., Zhang T., Zhu L., Sun L., Shao G., Gao J. (2023). Recent advances of using exosomes as diagnostic markers and targeting carriers for cardiovascular disease. Mol. Pharm. 20 (9), 4354–4372. 10.1021/acs.molpharmaceut.3c00268 [DOI] [PubMed] [Google Scholar]
- Liu C., Yang Y., Wu Y. (2018). Recent advances in exosomal protein detection via liquid biopsy biosensors for cancer screening, diagnosis, and prognosis. AAPS J. 20 (2), 41. 10.1208/s12248-018-0201-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Wang J., Wang P., Zhong L., Wang S., Feng Q., et al. (2022). Hypoxia-pretreated mesenchymal stem cell-derived exosomes-loaded low-temperature extrusion 3D-printed implants for neural regeneration after traumatic brain injury in canines. Front. Bioeng. Biotechnol. 10, 1025138. 10.3389/fbioe.2022.1025138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. Y., Feng Y. H., Feng Q. B., Zhang J. Y., Zhong L., Liu P., et al. (2023). Low-temperature 3D-printed collagen/chitosan scaffolds loaded with exosomes derived from neural stem cells pretreated with insulin growth factor-1 enhance neural regeneration after traumatic brain injury. Neural Regen. Res. 18 (9), 1990–1998. 10.4103/1673-5374.366497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wen J., Huang W. (2021). Exosomes in nasopharyngeal carcinoma. Clin. Chim. Acta 523, 355–364. 10.1016/j.cca.2021.10.013 [DOI] [PubMed] [Google Scholar]
- Logozzi M., Angelini D. F., Giuliani A., Mizzoni D., Di Raimo R., Maggi M., et al. (2019). Increased plasmatic levels of PSA-expressing exosomes distinguish prostate cancer patients from benign prostatic hyperplasia: a prospective study. Cancers (Basel) 11 (10), 1449. 10.3390/cancers11101449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logozzi M., Orefice N. S., Di Raimo R., Mizzoni D., Fais S. (2023). The importance of detecting, quantifying, and characterizing exosomes as a new diagnostic/prognostic approach for tumor patients. Cancers (Basel) 15 (11), 2878. 10.3390/cancers15112878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscocco F., Visani G., Galimberti S., Curti A., Isidori A. (2019). BCR-ABL independent mechanisms of resistance in chronic myeloid leukemia. Front. Oncol. 9, 939. 10.3389/fonc.2019.00939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Zhang Y., Yang X., Zhao H. (2023). Harnessing exosomes as cutting-edge drug delivery systems for revolutionary osteoarthritis therapy. Biomed. Pharmacother. 165, 115135. 10.1016/j.biopha.2023.115135 [DOI] [PubMed] [Google Scholar]
- Luga V., Zhang L., Viloria-Petit A. M., Ogunjimi A. A., Inanlou M. R., Chiu E., et al. (2012). Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 151 (7), 1542–1556. 10.1016/j.cell.2012.11.024 [DOI] [PubMed] [Google Scholar]
- Luo M., Luan X., Jiang G., Yang L., Yan K., Li S., et al. (2023). The dual effects of exosomes on glioma: a comprehensive review. J. Cancer 14 (14), 2707–2719. 10.7150/jca.86996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X., An M., Cuneo K. C., Lubman D. M., Li L. (2018). High-performance chemical isotope labeling liquid chromatography mass spectrometry for exosome metabolomics. Anal. Chem. 90 (14), 8314–8319. 10.1021/acs.analchem.8b01726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Li C., Huang Y., Wang Y., Xia X., Zheng J. C. (2019). Exosomes released from neural progenitor cells and induced neural progenitor cells regulate neurogenesis through miR-21a. Cell Commun. Signal 17 (1), 96. 10.1186/s12964-019-0418-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud A. M., Childs D. S., Ahmed M. E., Tuba Kendi A., Johnson G. B., Orme J. J., et al. (2023). Treatment modalities and survival outcomes in prostate cancer parenchymal brain metastasis. Prostate 84, 237–244. 10.1002/pros.24643 [DOI] [PubMed] [Google Scholar]
- Maia J., Caja S., Strano Moraes M. C., Couto N., Costa-Silva B. (2018). Exosome-based cell-cell communication in the tumor microenvironment. Front. Cell Dev. Biol. 6, 18. 10.3389/fcell.2018.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maji S., Chaudhary P., Akopova I., Nguyen P. M., Hare R. J., Gryczynski I., et al. (2017). Exosomal annexin II promotes angiogenesis and breast cancer metastasis. Mol. Cancer Res. 15 (1), 93–105. 10.1158/1541-7786.MCR-16-0163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malekian F., Shamsian A., Kodam S. P., Ullah M. (2023). Exosome engineering for efficient and targeted drug delivery: current status and future perspective. J. Physiol. 601 (22), 4853–4872. 10.1113/JP282799 [DOI] [PubMed] [Google Scholar]
- Mallawaaratchy D. M., Hallal S., Russell B., Ly L., Ebrahimkhani S., Wei H., et al. (2017). Comprehensive proteome profiling of glioblastoma-derived extracellular vesicles identifies markers for more aggressive disease. J. Neurooncol 131 (2), 233–244. 10.1007/s11060-016-2298-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. J. (2020). Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. J. 55 (4), 2000607. 10.1183/13993003.00607-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu M., Martin-Jaular L., Lavieu G., Thery C. (2019). Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 21 (1), 9–17. 10.1038/s41556-018-0250-9 [DOI] [PubMed] [Google Scholar]
- Maumus M., Rozier P., Boulestreau J., Jorgensen C., Noel D. (2020). Mesenchymal stem cell-derived extracellular vesicles: opportunities and challenges for clinical translation. Front. Bioeng. Biotechnol. 8, 997. 10.3389/fbioe.2020.00997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAndrews K. M., Kalluri R. (2019). Mechanisms associated with biogenesis of exosomes in cancer. Mol. Cancer 18 (1), 52. 10.1186/s12943-019-0963-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaomiao S., Xiaoqian W., Yuwei S., Chao C., Chenbo Y., Yinghao L., et al. (2023). Cancer-associated fibroblast-derived exosome microRNA-21 promotes angiogenesis in multiple myeloma. Sci. Rep. 13 (1), 9671. 10.1038/s41598-023-36092-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. I., Ma J., Carter C. L., Loudig O. (2022). Circulating exosome cargoes contain functionally diverse cancer biomarkers: from biogenesis and function to purification and potential translational utility. Cancers (Basel) 14 (14), 3350. 10.3390/cancers14143350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moitra K., Lou H., Dean M. (2011). Multidrug efflux pumps and cancer stem cells: insights into multidrug resistance and therapeutic development. Clin. Pharmacol. Ther. 89 (4), 491–502. 10.1038/clpt.2011.14 [DOI] [PubMed] [Google Scholar]
- Mukhtar M., Sargazi S., Barani M., Madry H., Rahdar A., Cucchiarini M. (2021). Application of nanotechnology for sensitive detection of low-abundance single-nucleotide variations in genomic DNA: a review. Nanomater. (Basel) 11 (6), 1384. 10.3390/nano11061384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi V. N., Perkins R. B., Sy S., Kim J. J. (2022). Cost-effectiveness analysis of the 2019 American Society for Colposcopy and Cervical Pathology Risk-Based Management Consensus Guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Am. J. Obstet. Gynecol. 226 (2), 228.e1–228.e9. 10.1016/j.ajog.2021.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan A., Iordanskiy S., Das R., Van Duyne R., Santos S., Jaworski E., et al. (2013). Exosomes derived from HIV-1-infected cells contain trans-activation response element RNA. J. Biol. Chem. 288 (27), 20014–20033. 10.1074/jbc.M112.438895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nik Mohamed Kamal N. N. S., Shahidan W. N. S. (2021). Salivary exosomes: from waste to promising periodontitis treatment. Front. Physiol. 12, 798682. 10.3389/fphys.2021.798682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu L. M., Song X. G., Wang N., Xue L. L., Song X. E., Xie L. (2019). Tumor-derived exosomal proteins as diagnostic biomarkers in non-small cell lung cancer. Cancer Sci. 110 (1), 433–442. 10.1111/cas.13862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoye I. S., Coomes S. M., Pelly V. S., Czieso S., Papayannopoulos V., Tolmachova T., et al. (2014). MicroRNA-Containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity 41 (3), 503. 10.1016/j.immuni.2014.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostenfeld M. S., Jeppesen D. K., Laurberg J. R., Boysen A. T., Bramsen J. B., Primdal-Bengtson B., et al. (2014). Cellular disposal of miR23b by RAB27-dependent exosome release is linked to acquisition of metastatic properties. Cancer Res. 74 (20), 5758–5771. 10.1158/0008-5472.Can-13-3512 [DOI] [PubMed] [Google Scholar]
- Palmisano G., Jensen S. S., Le Bihan M. C., Laine J., McGuire J. N., Pociot F., et al. (2012). Characterization of membrane-shed microvesicles from cytokine-stimulated β-cells using proteomics strategies. Mol. Cell Proteomics 11 (8), 230–243. 10.1074/mcp.M111.012732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Li Y., Dong W., Jiang B., Yu Y., Chen Y. (2023). Role of nano-hydrogels coated exosomes in bone tissue repair. Front. Bioeng. Biotechnol. 11, 1167012. 10.3389/fbioe.2023.1167012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel G. K., Khan M. A., Zubair H., Srivastava S. K., Khushman M., Singh S., et al. (2019). Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 9 (1), 5335. 10.1038/s41598-019-41800-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patolsky F., Weizmann Y., Katz E., Willner I. (2003). Magnetically amplified DNA assays (MADA): sensing of viral DNA and single-base mismatches by using nucleic acid modified magnetic particles. Angew. Chem. Int. Ed. Engl. 42 (21), 2372–2376. 10.1002/anie.200250379 [DOI] [PubMed] [Google Scholar]
- Pols M. S., Klumperman J. (2009). Trafficking and function of the tetraspanin CD63. Exp. Cell Res. 315 (9), 1584–1592. 10.1016/j.yexcr.2008.09.020 [DOI] [PubMed] [Google Scholar]
- Pu Q., Chai J., Chen L., Liu C., Yang C., Huang Y., et al. (2022). Exosome miRNA expression in umbilical cord blood of high-parity sows regulates their reproductive potential. Anim. (Basel) 12 (18), 2456. 10.3390/ani12182456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Z., Travanty E. A., Oko L., Edeen K., Berglund A., Wang J., et al. (2013). Innate immune response of human alveolar type II cells infected with severe acute respiratory syndrome-coronavirus. Am. J. Respir. Cell Mol. Biol. 48 (6), 742–748. 10.1165/rcmb.2012-0339OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjbaran A., Latifi Z., Nejabati H. R., Abroon S., Mihanfar A., Sadigh A. R., et al. (2019). Exosome-based intercellular communication in female reproductive microenvironments. J. Cell Physiol. 234 (11), 19212–19222. 10.1002/jcp.28668 [DOI] [PubMed] [Google Scholar]
- Regev-Rudzki N., Wilson D. W., Carvalho T. G., Sisquella X., Coleman B. M., Rug M., et al. (2013). Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell 153 (5), 1120–1133. 10.1016/j.cell.2013.04.029 [DOI] [PubMed] [Google Scholar]
- Rozmyslowicz T., Majka M., Kijowski J., Murphy S. L., Conover D. O., Poncz M., et al. (2003). Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. Aids 17 (1), 33–42. 10.1097/00002030-200301030-00006 [DOI] [PubMed] [Google Scholar]
- Saad M. G., Beyenal H., Dong W. J. (2021). Exosomes as powerful engines in cancer: isolation, characterization and detection techniques. Biosens. (Basel) 11 (12), 518. 10.3390/bios11120518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi S., Tehrani F. R., Tahmasebi S., Shafiee A., Hashemi S. M. (2023). Exosome engineering in cell therapy and drug delivery. Inflammopharmacology 31 (1), 145–169. 10.1007/s10787-022-01115-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakwe A. M., Koumangoye R., Guillory B., Ochieng J. (2011). Annexin A6 contributes to the invasiveness of breast carcinoma cells by influencing the organization and localization of functional focal adhesions. Exp. Cell Res. 317 (6), 823–837. 10.1016/j.yexcr.2010.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salciccia S., Frisenda M., Bevilacqua G., Gobbi L., Bucca B., Moriconi M., et al. (2023). Exosome analysis in prostate cancer: how they can improve biomarkers' performance. Curr. Issues Mol. Biol. 45 (7), 6085–6096. 10.3390/cimb45070384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho-Albero M., Navascues N., Mendoza G., Sebastian V., Arruebo M., Martin-Duque P., et al. (2019). Exosome origin determines cell targeting and the transfer of therapeutic nanoparticles towards target cells. J. Nanobiotechnology 17 (1), 16. 10.1186/s12951-018-0437-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Ros J., Romero-Garcia N., Mas-Bargues C., Monleon D., Gordevicius J., Brooke R. T., et al. (2022). Small extracellular vesicles from young adipose-derived stem cells prevent frailty, improve health span, and decrease epigenetic age in old mice. Sci. Adv. 8 (42), eabq2226. 10.1126/sciadv.abq2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavo M. P., Depalo N., Tutino V., De Nunzio V., Ingrosso C., Rizzi F., et al. (2020). Exosomes for diagnosis and therapy in gastrointestinal cancers. Int. J. Mol. Sci. 21 (1), 367. 10.3390/ijms21010367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D. (2020). A brief history of adherons: the discovery of brain exosomes. Int. J. Mol. Sci. 21 (20), 7673. 10.3390/ijms21207673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzenbach H., Gahan P. B. (2021). Exosomes in immune regulation. Noncoding RNA 7 (1), 4. 10.3390/ncrna7010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Z., Wanyan P., Wang M., Zhang B., Cui X., Wang X. (2023). Stem cell-derived exosomes for traumatic spinal cord injury: a systematic review and network meta-analysis based on a rat model. Cytotherapy 26, 1–10. 10.1016/j.jcyt.2023.09.002 [DOI] [PubMed] [Google Scholar]
- Sharma P., Mesci P., Carromeu C., McClatchy D. R., Schiapparelli L., Yates J. R., 3rd, et al. (2019). Exosomes regulate neurogenesis and circuit assembly. Proc. Natl. Acad. Sci. U. S. A. 116 (32), 16086–16094. 10.1073/pnas.1902513116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Salomon C. (2020). Techniques associated with exosome isolation for biomarker development: liquid biopsies for ovarian cancer detection. Methods Mol. Biol. 2055, 181–199. 10.1007/978-1-4939-9773-2_8 [DOI] [PubMed] [Google Scholar]
- Sharma S., Zuniga F., Rice G. E., Perrin L. C., Hooper J. D., Salomon C. (2017). Tumor-derived exosomes in ovarian cancer - liquid biopsies for early detection and real-time monitoring of cancer progression. Oncotarget 8 (61), 104687–104703. 10.18632/oncotarget.22191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shegekar T., Vodithala S., Juganavar A. (2023). The emerging role of liquid biopsies in revolutionising cancer diagnosis and therapy. Cureus 15 (8), e43650. 10.7759/cureus.43650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Xue C., Li X., Ba L., Gu J., Sun Z., et al. (2019). Effects of gastric cancer cell-derived exosomes on the immune regulation of mesenchymal stem cells by the NF-kB signaling pathway. Stem Cells Dev. 28 (7), 464–476. 10.1089/scd.2018.0125 [DOI] [PubMed] [Google Scholar]
- Shenoda B. B., Ajit S. K. (2016). Modulation of immune responses by exosomes derived from antigen-presenting cells. Clin. Med. Insights Pathol. 9 (1), 1–8. 10.4137/CPath.S39925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si Q., Wu L., Pang D., Jiang P. (2023). Exosomes in brain diseases: pathogenesis and therapeutic targets. MedComm 4 (3), e287. 10.1002/mco2.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva P., Mendoza P., Rivas S., Diaz J., Moraga C., Quest A. F., et al. (2016). Hypoxia promotes Rab5 activation, leading to tumor cell migration, invasion and metastasis. Oncotarget 7 (20), 29548–29562. 10.18632/oncotarget.8794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. A., Lam A. K. (2018). Liquid biopsy for investigation of cancer DNA in esophageal adenocarcinoma: cell-free plasma DNA and exosome-associated DNA. Methods Mol. Biol. 1756, 187–194. 10.1007/978-1-4939-7734-5_17 [DOI] [PubMed] [Google Scholar]
- Sohn W., Kim J., Kang S. H., Yang S. R., Cho J. Y., Cho H. C., et al. (2015). Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma. Exp. Mol. Med. 47 (9), e184. 10.1038/emm.2015.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A., Amreddy N., Razaq M., Towner R., Zhao Y. D., Ahmed R. A., et al. (2018). Exosomes as theranostics for lung cancer. Adv. Cancer Res. 139, 1–33. 10.1016/bs.acr.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbichler T. B., Dudas J., Skvortsov S., Ganswindt U., Riechelmann H., Skvortsova I. I. (2019). Therapy resistance mediated by exosomes. Mol. Cancer 18 (1), 58. 10.1186/s12943-019-0970-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimachi K., Matsumura T., Hirata H., Uchi R., Ueda M., Ueo H., et al. (2015). Identification of a bona fide microRNA biomarker in serum exosomes that predicts hepatocellular carcinoma recurrence after liver transplantation. Br. J. Cancer 112 (3), 532–538. 10.1038/bjc.2014.621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W., Xing C., Zhao L., Zhao P., Yang G., Yuan L. (2020). Ultrasound assisted exosomal delivery of tissue responsive mRNA for enhanced efficacy and minimized off-target effects. Mol. Ther. Nucleic Acids 20, 558–567. 10.1016/j.omtn.2020.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro H., Umezu T., Ohyashiki K., Hirano T., Ohyashiki J. H. (2013). Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J. Biol. Chem. 288 (48), 34343–34351. 10.1074/jbc.M113.480822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai Y. L., Chen K. C., Hsieh J. T., Shen T. L. (2018). Exosomes in cancer development and clinical applications. Cancer Sci. 109 (8), 2364–2374. 10.1111/cas.13697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka M. C., Quintana F. J. (2017). Tolerogenic dendritic cells. Semin. Immunopathol. 39 (2), 113–120. 10.1007/s00281-016-0587-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H., Hosein P. J. (2023). Detection and therapeutic implications of homologous recombination repair deficiency in pancreatic cancer: a narrative review. J. Gastrointest. Oncol. 14 (5), 2249–2259. 10.21037/jgo-23-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S., Cheng J., Yao Y., Lou C., Wang L., Huang X., et al. (2020). Combination of four serum exosomal MiRNAs as novel diagnostic biomarkers for early-stage gastric cancer. Front. Genet. 11, 237. 10.3389/fgene.2020.00237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. D., Gercel-Taylor C. (2008). MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 110 (1), 13–21. 10.1016/j.ygyno.2008.04.033 [DOI] [PubMed] [Google Scholar]
- Thakur B. K., Zhang H., Becker A., Matei I., Huang Y., Costa-Silva B., et al. (2014). Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 24 (6), 766–769. 10.1038/cr.2014.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thill M., Kolberg-Liedtke C., Albert U. S., Banys-Paluchowski M., Bauerfeind I., Blohmer J. U., et al. (2023). AGO recommendations for the diagnosis and treatment of patients with locally advanced and metastatic breast cancer: update 2023. Breast Care (Basel) 18 (4), 306–315. 10.1159/000531579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thind A., Wilson C. (2016). Exosomal miRNAs as cancer biomarkers and therapeutic targets. J. Extracell. Vesicles 5, 31292. 10.3402/jev.v5.31292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trams E. G., Lauter C. J., Salem N., Jr., Heine U. (1981). Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta 645 (1), 63–70. 10.1016/0005-2736(81)90512-5 [DOI] [PubMed] [Google Scholar]
- Umezu T., Tadokoro H., Azuma K., Yoshizawa S., Ohyashiki K., Ohyashiki J. H. (2014). Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood 124 (25), 3748–3757. 10.1182/blood-2014-05-576116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcz G., Galamb O., Krenacs T., Spisak S., Kalmar A., Patai A. V., et al. (2016). Exosomes in colorectal carcinoma formation: ALIX under the magnifying glass. Mod. Pathol. 29 (8), 928–938. 10.1038/modpathol.2016.72 [DOI] [PubMed] [Google Scholar]
- Wang H., Hou L., Li A., Duan Y., Gao H., Song X. (2014). Expression of serum exosomal microRNA-21 in human hepatocellular carcinoma. Biomed. Res. Int. 2014, 864894. 10.1155/2014/864894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Yang L. (2023). The role of exosomes in central nervous system tissue regeneration and repair. Biomed. Mater 18 (5), 052003. 10.1088/1748-605X/ace39c [DOI] [PubMed] [Google Scholar]
- Wang J. C., Bégin L. R., Bérube N. G., Chevalier S., Aprikian A. G., Gourdeau H., et al. (2007). Down-regulation of CD9 expression during prostate carcinoma progression is associated with CD9 mRNA modifications. Clin. Cancer Res. 13 (8), 2354–2361. 10.1158/1078-0432.Ccr-06-1692 [DOI] [PubMed] [Google Scholar]
- Wang L., Skotland T., Berge V., Sandvig K., Llorente A. (2017). Exosomal proteins as prostate cancer biomarkers in urine: from mass spectrometry discovery to immunoassay-based validation. Eur. J. Pharm. Sci. 98, 80–85. 10.1016/j.ejps.2016.09.023 [DOI] [PubMed] [Google Scholar]
- Wang M., Hu D., Yang Y., Shi K., Li J., Liu Q., et al. (2023a). Enhanced chemo-immunotherapy strategy utilizing injectable thermosensitive hydrogel for the treatment of diffuse peritoneal metastasis in advanced colorectal cancer. Adv. Sci. (Weinh) 10, e2303819. 10.1002/advs.202303819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Wang Y., Tian X., Wang Q., Huang H., Lu X., et al. (2023b). Diagnostic and predictive value of liquid biopsy-derived exosome miR-21 for breast cancer: a systematic review and meta-analysis. Expert Rev. Mol. Diagn 23 (4), 315–324. 10.1080/14737159.2023.2195552 [DOI] [PubMed] [Google Scholar]
- Wang T. W., Chao H. S., Chiu H. Y., Lu C. F., Liao C. Y., Lee Y., et al. (2023). Radiomics of metastatic brain tumor as a predictive image biomarker of progression-free survival in patients with non-small-cell lung cancer with brain metastasis receiving tyrosine kinase inhibitors. Transl. Oncol. 39, 101826. 10.1016/j.tranon.2023.101826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Du X., Lian W., Chen J., Hong C., Li L., et al. (2023). A novel disulfidptosis-associated expression pattern in breast cancer based on machine learning. Front. Genet. 14, 1193944. 10.3389/fgene.2023.1193944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside T. L. (2017). The effect of tumor-derived exosomes on immune regulation and cancer immunotherapy. Future Oncol. 13 (28), 2583–2592. 10.2217/fon-2017-0343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf A. M. D., Oeffinger K. C., Shih T. Y., Walter L. C., Church T. R., Fontham E. T. H., et al. (2023). Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J. Clin. 68, 250–281. 10.3322/caac.21457 [DOI] [PubMed] [Google Scholar]
- Wu D., Chang X., Tian J., Kang L., Wu Y., Liu J., et al. (2021). Bone mesenchymal stem cells stimulation by magnetic nanoparticles and a static magnetic field: release of exosomal miR-1260a improves osteogenesis and angiogenesis. J. Nanobiotechnology 19 (1), 209. 10.1186/s12951-021-00958-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Wang G., Hu W., Yao Y., Yu X. F. (2019). Emerging roles and therapeutic value of exosomes in cancer metastasis. Mol. Cancer 18 (1), 53. 10.1186/s12943-019-0964-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. W., Li L., Wang Y., Xiao Z. (2019). CTL-derived exosomes enhance the activation of CTLs stimulated by low-affinity peptides. Front. Immunol. 10, 1274. 10.3389/fimmu.2019.01274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Liu Y., Cao Y., Liu Z. (2022). Engineering macrophage exosome disguised biodegradable nanoplatform for enhanced sonodynamic therapy of glioblastoma. Adv. Mater 34 (15), e2110364. 10.1002/adma.202110364 [DOI] [PubMed] [Google Scholar]
- Xia Y., Hu X., Di K., Liu C., Tan T., Lin Y., et al. (2020). Combined detection of exosome concentration and tumor markers in gastric cancer. J. Biomed. Nanotechnol. 16 (2), 252–258. 10.1166/jbn.2020.2887 [DOI] [PubMed] [Google Scholar]
- Xiao L., Hareendran S., Loh Y. P. (2021). Function of exosomes in neurological disorders and brain tumors. Extracell. Vesicles Circ. Nucl. Acids 2, 55–79. 10.20517/evcna.2021.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X. (2015). Screening, dignosis and follow-up in lung cancer in early stage. Zhonghua Yi Xue Za Zhi 95 (43), 3481–3483. [PubMed] [Google Scholar]
- Yang M., Chen J. Q., Su F., Yu B., Su F. X., Lin L., et al. (2011). Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol. Cancer 10, 117. 10.1186/1476-4598-10-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. J., Wang D. D., Li J., Xu H. Z., Shen H. Y., Chen X., et al. (2017). Predictive role of GSTP1-containing exosomes in chemotherapy-resistant breast cancer. Gene 623, 5–14. 10.1016/j.gene.2017.04.031 [DOI] [PubMed] [Google Scholar]
- Yi X., Chen J., Huang D., Feng S., Yang T., Li Z., et al. (2022). Current perspectives on clinical use of exosomes as novel biomarkers for cancer diagnosis. Front. Oncol. 12, 966981. 10.3389/fonc.2022.966981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E., Edwards L., Singh R. (2023). The role of artificial intelligence in colorectal cancer screening: lesion detection and lesion characterization. Cancers (Basel) 15 (21), 5126. 10.3390/cancers15215126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D., Li Y., Wang M., Gu J., Xu W., Cai H., et al. (2022). Exosomes as a new frontier of cancer liquid biopsy. Mol. Cancer 21 (1), 56. 10.1186/s12943-022-01509-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Hurley J., Roberts D., Chakrabortty S. K., Enderle D., Noerholm M., et al. (2021). Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann. Oncol. 32 (4), 466–477. 10.1016/j.annonc.2021.01.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y., Fu B. M. (2020). Resistance mechanisms of anti-angiogenic therapy and exosomes-mediated revascularization in cancer. Front. Cell Dev. Biol. 8, 610661. 10.3389/fcell.2020.610661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Guan J., Niu X., Hu G., Guo S., Li Q., et al. (2015). Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J. Transl. Med. 13, 49. 10.1186/s12967-015-0417-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Cheng K. (2023). Stem cell-derived exosome versus stem cell therapy. Nat. Rev. Bioeng. 1, 608–609. 10.1038/s44222-023-00064-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., He F., Gao L., Cong M., Sun J., Xu J., et al. (2021). Engineering exosome-like nanovesicles derived from Asparagus cochinchinensis can inhibit the proliferation of hepatocellular carcinoma cells with better safety profile. Int. J. Nanomedicine 16, 1575–1586. 10.2147/IJN.S293067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Wan L., Li R., Li X., Zhu T., Lu H. (2023). Engineered exosomes for tissue regeneration: from biouptake, functionalization and biosafety to applications. Biomater. Sci. 11 (22), 7247–7267. 10.1039/d3bm01169k [DOI] [PubMed] [Google Scholar]
- Zhang W., Peng P., Shen K. (2016). Role of exosome shuttle RNA in cell-to-cell communication. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 38 (4), 480–483. 10.3881/j.issn.1000-503X.2016.04.020 [DOI] [PubMed] [Google Scholar]
- Zhang W., Pham T. H., Wang D. H. (2021). A "clue" to improving liquid biopsies for cancer: microfluidic multiparametric exosome analysis. Clin. Chem. 67 (2), 335–337. 10.1093/clinchem/hvaa209 [DOI] [PubMed] [Google Scholar]
- Zhang X. C., Lu S., Zhang L., Wang C. L., Cheng Y., Li G. D., et al. (2013). Consensus on dignosis for ALK positive non-small cell lung cancer in China, the 2013 version. Zhonghua Bing Li Xue Za Zhi 42 (6), 402–406. 10.3760/cma.j.issn.0529-5807.2013.06.012 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Ali A., Xie J. (2023). Detection of clinically important BRCA gene mutations in ovarian cancer patients using next generation sequencing analysis. Am. J. Cancer Res. 13 (10), 5005–5020. [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Yang J., Jiao J., Wang X., Zhao Y., Zhang L. (2023). Biomedical applications of artificial exosomes for intranasal drug delivery. Front. Bioeng. Biotechnol. 11, 1271489. 10.3389/fbioe.2023.1271489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Fu L., Zou H., He Y., Pan Y., Ye L., et al. (2023). Optogenetic engineered umbilical cord MSC-derived exosomes for remodeling of the immune microenvironment in diabetic wounds and the promotion of tissue repair. J. Nanobiotechnology 21 (1), 176. 10.1186/s12951-023-01886-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Liu L., Sun R., Cui G., Guo S., Han S., et al. (2022). Exosomes in cancer immunoediting and immunotherapy. Asian J. Pharm. Sci. 17 (2), 193–205. 10.1016/j.ajps.2021.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Tao M., Wei M., Du S., Wang H., Wang X. (2019). Mesenchymal stem cells derived exosomal miR-323-3p promotes proliferation and inhibits apoptosis of cumulus cells in polycystic ovary syndrome (PCOS). Artif. Cells Nanomed Biotechnol. 47 (1), 3804–3813. 10.1080/21691401.2019.1669619 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Xu L., Ma W., Wang L., Kuang H., Xu C., et al. (2014). Shell-engineered chiroplasmonic assemblies of nanoparticles for zeptomolar DNA detection. Nano Lett. 14 (7), 3908–3913. 10.1021/nl501166m [DOI] [PubMed] [Google Scholar]
- Zhong L., Wang J., Wang P., Liu X., Liu P., Cheng X., et al. (2023). Neural stem cell-derived exosomes and regeneration: cell-free therapeutic strategies for traumatic brain injury. Stem Cell Res. Ther. 14 (1), 198. 10.1186/s13287-023-03409-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K., Li Z. Z., Cai Z. M., Zhong N. N., Cao L. M., Huo F. Y., et al. (2023). Nanotheranostics in cancer lymph node metastasis: the long road ahead. Pharmacol. Res. 198, 106989. 10.1016/j.phrs.2023.106989 [DOI] [PubMed] [Google Scholar]
- Zhou W., Fong M. Y., Min Y., Somlo G., Liu L., Palomares M. R., et al. (2014). Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 25 (4), 501–515. 10.1016/j.ccr.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Zhang Y., Gong H., Luo S., Cui Y. (2021). The role of exosomes and their applications in cancer. Int. J. Mol. Sci. 22 (22), 12204. 10.3390/ijms222212204 [DOI] [PMC free article] [PubMed] [Google Scholar]