Fig. 1. Cryo-EM analysis of ribosomes from cold-adapted bacteria identifies a new ribosome hibernation factor, Balon.
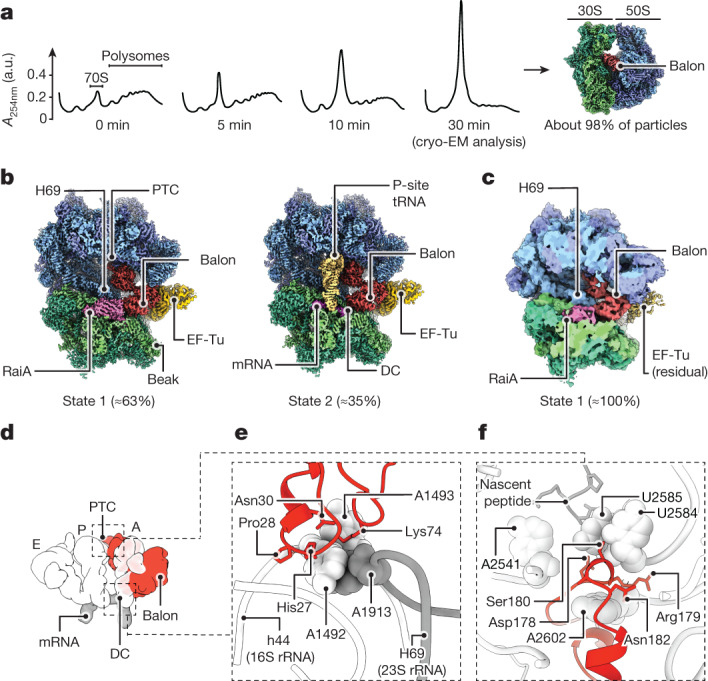
a, Polysome profiling in sucrose gradients shows an accumulation of monomeric ribosomes during the first 30 min of response to ice treatment of P. urativorans cells. Absorbance (A) was measured at 260 nm in arbitrary units (a.u.). b, Cryo-EM maps at 2.6 Å resolution depicting the two most prevalent states of ribosomes isolated from ice-treated bacteria P. urativorans. State 1 consists of ribosomes bound to a previously uncharacterized protein, Balon, and the hibernation factor RaiA. State 2 represents ribosomes bound to Balon, mRNA and P-site tRNA. Both states of the ribosome also show the presence of the elongation factor EF-Tu bound to Balon. PTC, peptidyl-transferase centre; DC, decoding centre. c, A cryo-EM map at 5 Å resolution depicting the most prevalent state of ribosomes isolated from P. urativorans during late stationary phase. d–f, Structural snapshots illustrating that Balon occupies ribosomal active centres and overlaps with several drug-binding sites. d, Superposition of Balon (red), tRNAs (white) and mRNA (grey) to compare ribosomal binding sites (A, P and E) of these molecules. e,f, Zoomed-in view of the decoding centre (e) and peptidyl-transferase centre of the ribosome (f), showing details of ribosome recognition by Balon.
