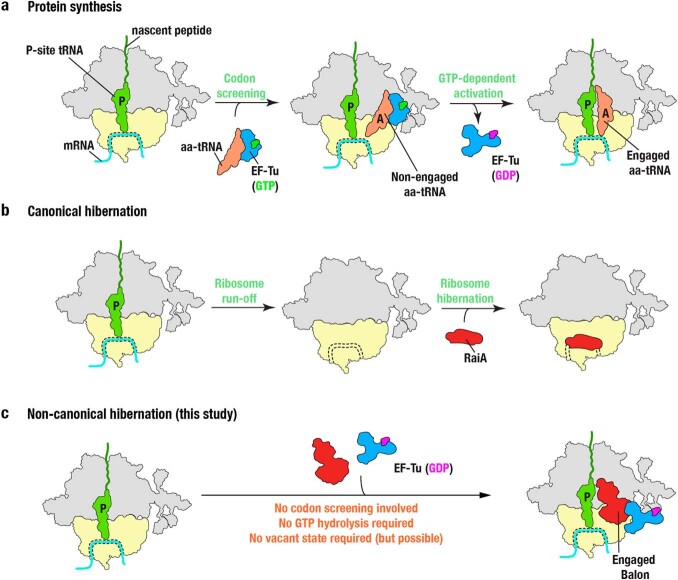
Extended Data Fig. 9. Comparison of Balon-mediated ribosome hibernation with the mechanisms of canonical protein synthesis and canonical ribosome hibernation.
(a) During normal protein synthesis, the elongation factor EF-Tu(GTP) delivers substrates for protein synthesis to the ribosomal A site and rapidly dissociates from the ribosome after GTP hydrolysis. (b) During starvation and stress, ribosomes become vacant after completing a cycle of protein synthesis, before binding to hibernation factors. EF-Tu is not required for this. (c) As in normal protein synthesis, Balon-mediated ribosome hibernation also involves the elongation factor EF-Tu and may occur while ribosomes remain associated with mRNAs and peptidyl-tRNA. However, unlike normal protein synthesis, this EF-Tu involvement appears to be antagonized by GTP, allowing only the GDP-bound form of EF-Tu to associate with hibernating ribosomes.