Fig. 5. Model of RNase R-mediated 30S subunit degradation.
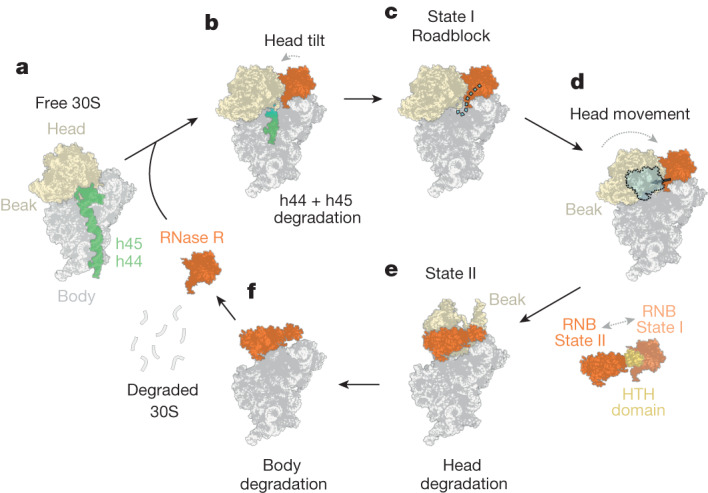
a, RNase R is a processive enzyme and at first targets the single-stranded 3′ end of the 16S rRNA. b, Binding of RNase R leads to head movements that open the mRNA channel and facilitate the degradation of the highly structured h45 and h44. c, During the degradation of part of h28, RNase R reaches the 30S head, which poses a steric hindrance; this allows the first energetically stable degradation intermediate (state I) to be captured by cryo-EM. d, Because half of h28 is degraded, the neck region becomes highly flexible, leading to movement of the 30S head. e, Eventually, the 30S head is further destabilized, leading to a marked rearrangement in which uS2 and uS3 are displaced, the head rotates by 160° and RNase R has moved position, using the HTH as an anchor. This state corresponds to the second stable degradation intermediate (state II), which was also visualized by cryo-EM. The rearrangements allow RNase R to continue the degradation of the 30S head. f, Eventually, the complete 30S subunit can be accessed and degraded, and RNase R can dissociate and rebind another 30S subunit to initiate a further round of degradation.
